July 23, 2020
CAR T-cell therapy - the process and side effects
CAR T-cell therapy is a novel form of immunotherapy against cancer. The innovative type of immunotherapy is to prime a patient’s immune system against cancer cells, that would normally be elusive for the body’s own defense mechanisms.
In order to achieve this, so-called T-cells are collected from a patient’s blood sample and then cultured in the laboratory.
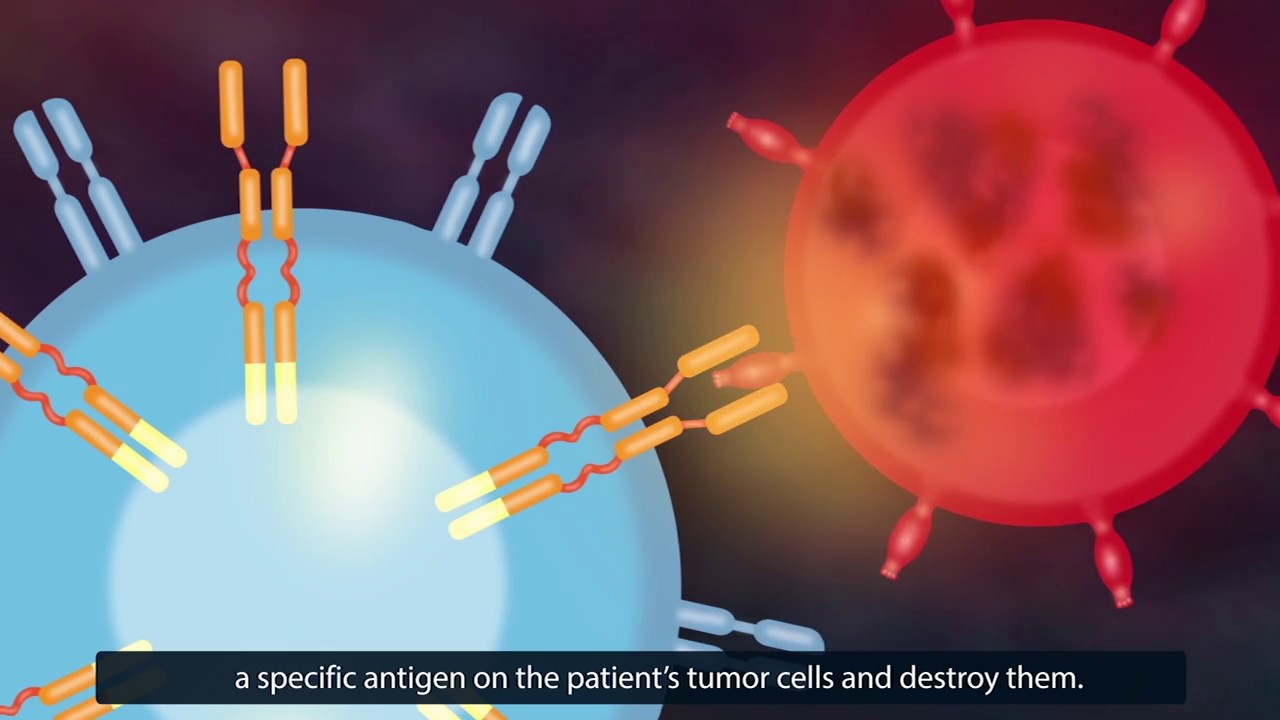
The T-cells are specially treated to produce specific sensors for the patient’s cancer cells, which are called chimeric antigen receptors (CAR). Subsequently, the resulting CAR T-cells are infused into the patient’s body, which is the reason for the alternate name T-cell transfer therapy.
When reintroduced into the patient’s body, CAR T-cells multiply, inform the immune system and attack tumor cells.
Application of the CAR T-cell therapy
Currently, the CAR T-cell therapy is successfully applied in the treatment of various types of blood cancers (leukemia) and lymph node cancers (lymphoma) such as diffuse large b-cell lymphoma, mantle cell lymphoma, follicular lymphoma, and other bone marrow cancers.
However, medical and pharmaceutical scientists in the oncology field are busy researching new applications in solid tumors such as breast cancer. As opposed to already approved cancer treatments (e. g. tisagenlecleucel in kymriah or axicabtagene ciloleucel in yescarta), during the development of new therapies mostly comparably small volumes of drug substances are handled.
Nonetheless, these have to be stored and shipped carefully. Diligent handling is imperative, and the sensitive matter does not allow for any glitches – neither during the production process in the lab nor on the final product’s way to the cancer center and into the patient. Delays and biocontamination can invalidate an entire batch – with massive financial and severe human consequences. After all, in this industry safety and secure transportation are fundamental.
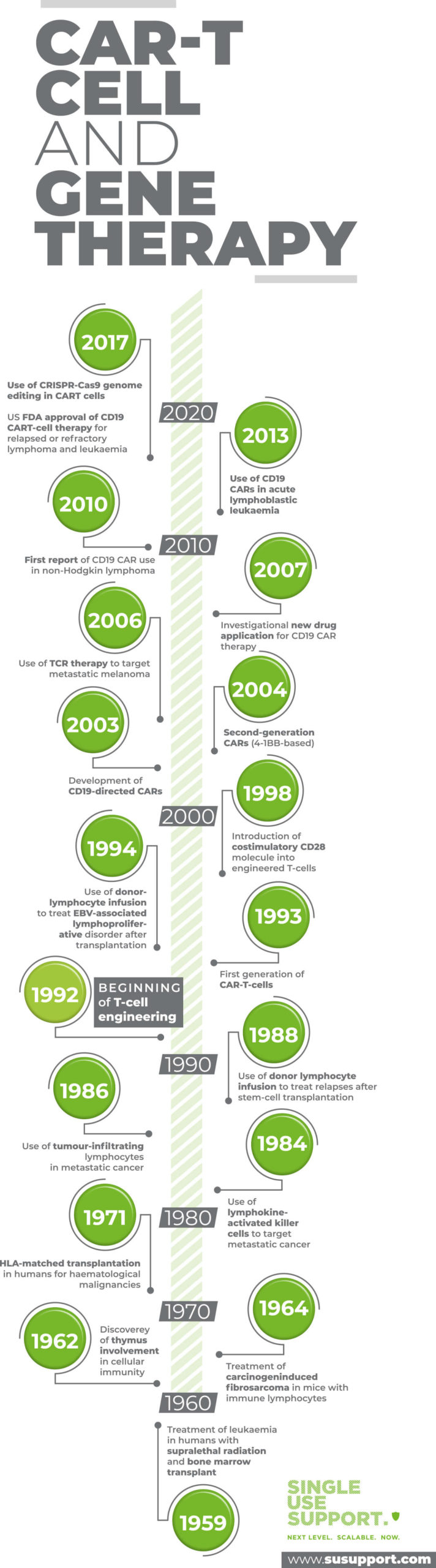
CAR T-cell therapy history
In 2010, the new therapy was successfully applied for the first time in adult patients: It is scientifically proven that two patients suffering from chronic lymphocytic leukemia (CLL) could be cured. And in 2012 in a pediatric setting, a girl suffering from Acute Lymphoblastic Leukemia (ALL) was treated successfully in the US. While the patient, who was 7 years at the time of treatment, reacted with severe side effects, today as a young adult she is considered to be cured.
In 2010, the new therapy was successfully applied for the first time in adult patients: It is scientifically proven that two patients suffering from chronic lymphocytic leukemia (CLL) could be cured. And in 2012 in a pediatric setting, a girl suffering from Acute Lymphoblastic Leukemia (ALL) was treated successfully in the US. While the patient, who was 7 years at the time of treatment, reacted with severe side effects, today as a young adult she is considered to be cured.
Currently, in the US and the European Union, five severe illnesses can be treated with the CAR T-cell therapy. 300 further applications – more than 100 of which are aimed against various tumor diseases – are being developed at present. In Germany, the University Hospital Würzburg is a pioneering institution with regards to CAR-T therapies and the experts there have been treating patients with CAR T-cell products approved by the food and drug administration (FDA) since 2016.
Apart from the treatment the institution also focuses on researching further fields of application of this specific personalized therapy, and in 2018, two myeloma patients could be treated successfully. Current findings show that the CAR T-cells remain in the circulatory system even after the tumor cells have disappeared and it is believed that they can attack again in case of a relapse.
Clinical trials in cancer care centers use CAR T-cell constructs to research further oncological application options. The cells required for the studies are usually modified at the study centers’ labs before being tested with patients who are not eligible for commercially approved CAR-T therapies, either because of their symptoms or other circumstances.
This development is just one of the signs of the change that the medical and pharmaceutical world are subjected to. Instead of producing blockbusters in vast quantities, the current trend goes towards producing small volumes of personalized agents on the basis of mutated cells. And those can easily be generated in a small lab instead of a giant pharmaceutical plant. The changing requirements regarding volumes and procedures bring with it the need for new and innovative processes that are, above all, flexible and agile. The integration of single use technology is one of them.

How CAR T-cell therapy works?
In this personalized therapy, the respective patient’s immune cells, the so-called T-cells, a type of white blood cell, are extracted. Those cells are responsible for your body’s immune response system. In their natural state they will not recognize cancer cells, which is why they have to be genetically modified before being reintroduced in the patient’s bloodstream in order to identify diseased or damaged cells and to destroy them. This is made possible by the antigen specific receptors the cells have developed during their processing at the lab.
This type of tumor treatment is nothing short of realizing an ancient dream: It works by using the patient’s own immune system – albeit technologically manipulated – to combat a deadly disease.
As the therapy needs to be tailored to each patient receiving CAR T-cells, it requires elaborate and costly procedures. The industry reacts to the challenges posed by the new approach by developing innovative processes and flexible systems, with the aim to make the medical dream come true and accessible for a wide range of patients.
Find out more about our technology!
For several types of cancer that do not respond well to traditional treatments, the CAR T-cell therapy proves very effective. However, each and every medicine carries the potential for serious side effects and this is true for CAR T-cell therapy as well. Therefore, CAR T-cells are administered in specialized medical centers with extensively trained caregivers. Additionally, patients are monitored for the following side effects closely during, and for several weeks after, receiving the treatment:
Cytokine release syndrome (CRS)
CAR T-cells may release large amounts of immune system activating substances called cytokines, which can lead to:
- headaches
- aches in extremities
- fever and chills
- fatigue
- dizziness
- nausea, vomiting, diarrhea
- difficulty in breathing
- low blood pressure
- elevated heart rate
Nervous system
Nervous system side effects are possible manifestation of side effects of CAR T-cell therapy:
- headaches
- altered consciousness
- confusion
- agitation
- difficulties with speaking and understanding
- loss of balance
- tremors
- seizures
Due to these possible neurologic difficulties, patients are advised to avoid dangerous activities such as driving, or operating heavy machinery, for several weeks after receiving CAR T-cell therapy.
Other serious side effects
- allergic reactions to the infusion
- abnormal levels of blood electrolytes
- weak immune system, elevated risk of serious infections
- decreased blood cell counts, potentially leading to infections, tiredness, bleeding, bruising or clotting disorders
Please consult a doctor for detailed information on side effects!
CAR T-cell treatment process & logistics offstage
While being a highly promising and effective treatment, the production of CAR T-cell therapeutics is a time consuming and laborious process.
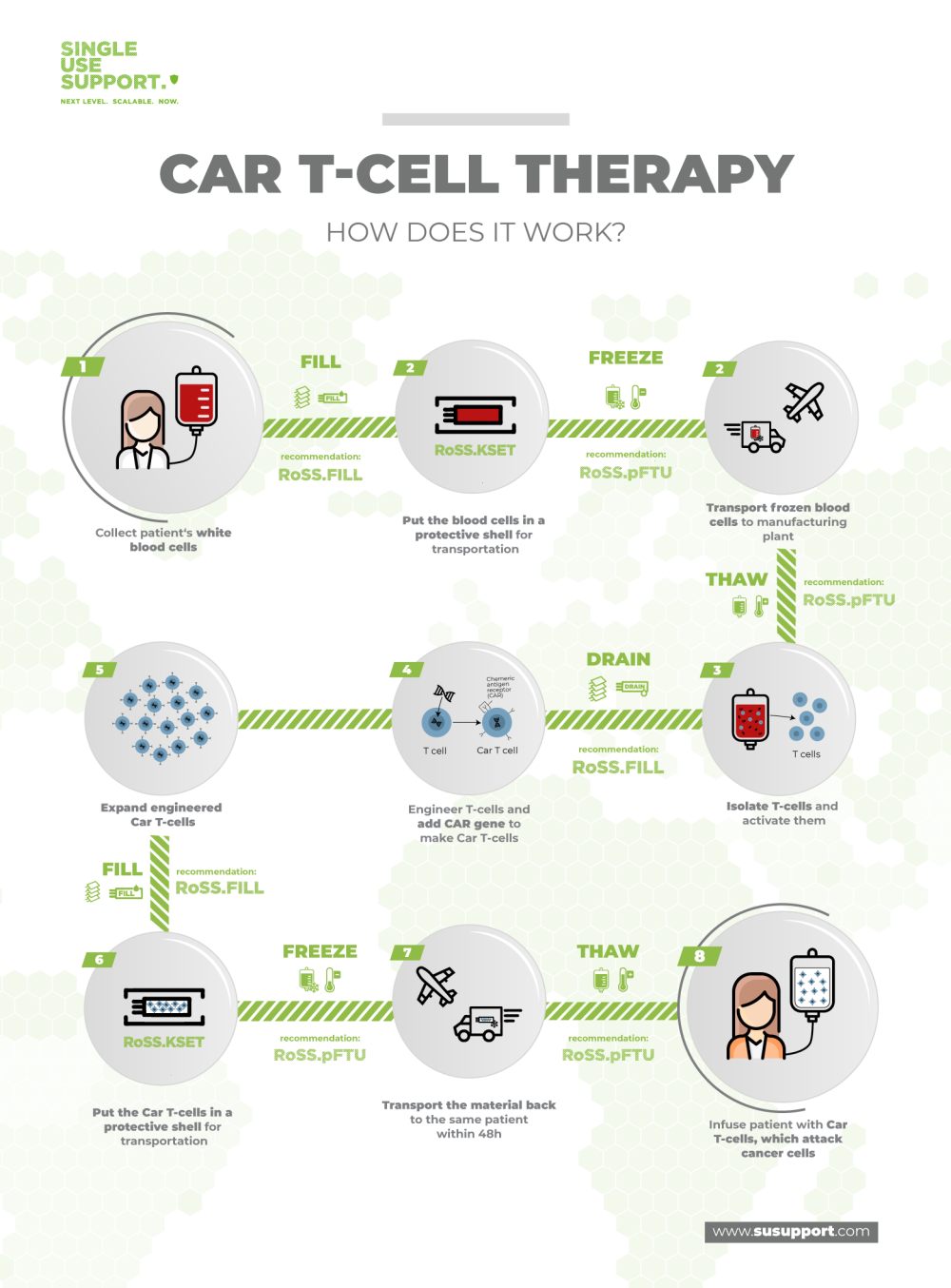
1. Collecting the T-cells from the patient
The first step is harvesting T-cells from the patient’s blood in a process termed leukapheresis. Blood is removed from the lying or sitting patient through an IV line and white blood cells, including T-cells, are separated. The treated blood is continually reinfused into the patient through a second IV line.
This apheresis procedure may take up to a few hours during which the patient needs to stay seated or lying. A common side effect is a tingling sensation or twitching. This condition is alleviated easily by giving calcium by mouth or via infusion.
2. Transporting the T-cells to the lab
Next, the harvested cells are prepared for their journey to the laboratory. They are filled into containers and put into protective shells to prevent any damage to the packaging. The blood cells are frozen and kept at low temperature during the transport to ensure their quality for the next steps
3. Production of individualized CAR T-cells
In the manufacturing plant, the T-cells are modified and equipped with the CAR gene. The resulting CAR T-cells are cultivated and expanded to produce large enough amounts to treat the patient. This step can take several weeks to be completed.
4. Transporting CAR T-cells to the patient
The modified T-cells are again packaged in sterile single use containers and protective shells. After freezing, the container is ready for the low-temperature transport to the medical center.
5. Infusion of the CAR T-cells
Prior to receiving the CAR T-cell infusion, the patient might undergo a mild cycle of chemotherapy to reduce the counts of other immune cells and give the CAR T-cells a better chance to be activated by cancer cells. Finally, the patient receives his own personalized infusion of CAR T-cells.
Challenges in the commercialization of CAR T-cell therapy
While CAR T-cell therapy is a highly promising cancer immunotherapy approach against hard-to-treat cancers, the broad commercialization of CAR T-cells is troublesome. The individualized process to generate CAR T-cells (one patient – one infusion) does not scale well economically. Moreover, the need for centralized laboratory facilities instead of manufacturing plants and the accompanying supply chains and transportation are considered bottlenecks.
For patients, factors other than production costs are of importance: the logistics and process quality of CAR T-cell generation. Vein to vein time is of utmost importance, since many cancers progress aggressively and patients perhaps cannot afford to wait out transporting delays and capacity shortages in supply chains.
Additionally, harvesting of T-cells and their subsequent modification are delicate processes involving sterile technique to avoid contamination and handling of miniscule volumes of highly valuable liquids without loss.
A very promising strategy to solve these logistics issues is the development of integrated platform solutions to handle freeze-thaw processes and manipulation of liquids.
Single Use Support’s contribution in the CAR T-cell therapy process
In order to guarantee the cells’ safety and sterility as well as assure the affordability of cancer research and development, innovative approaches and solutions are required. So far, the established systems, including those for freeze-thaw processes, are usually designed for vast amounts of drug substance. Drug substances that are still in the research or clinical trial phase are neglected.
But this is about to change: Single Use Support has developed an end-to-end process solution for cell & gene therapies that is scalable and geared towards use in labs where the handled volumes are usually as little as 0.03 to 0.2 fl oz (1 to 10mL). The system neither requires complicated adaptations nor extensions that might prove difficult to implement in a sterile setting and when time is of the essence. Quite on the contrary, the platform is easy to adapt and allows for the controlled filling as well as the monitored cultivation of cells.
A monitored freeze-thaw process assures an optimal product stability so that the high-quality liquids do not lose any of their efficacy when frozen. In order to also protect the valuable drug substances during shipping, they are sent on their way in the RoSS shell or its small volume version RoSS.KSET, both protective containers:
The filled and frozen single use bags are embedded in 3D foam to absorb external impacts and shocks, while a tamper-proof sleeve made of stainless steel offers additional stability and protection. This combination protects the sensitive single use bags from potential damages that can, in the worst case, cause the contamination of an entire batch and lead to dire consequences.

Seed Train Intensification Process by Single Use Support
Do you know about our innovative seed train intensification process already? Our process solution can be applied in different areas of Bioprocessing and Regenerative Medicine – Check it out!