November 25, 2021
Which countries is CAR T-cell therapy available in?
While CAR T-cell therapy, a novel type of cancer therapy, may still be in its early stages and showing certain limitations, it has been approved by the FDA and other institutions in the United States, Europe and other countries. Using modified chimeric anitgen receptor cells from the patient’s own blood to traget and kill cancer cells, this novel therapeutic approach has the potential to cure patients suffering from certain types of leukaemia and lymphoma. However, as of now, the geographical and socioeconomic distribution is still leaving space for improvement. Read the following article to find out why.
Success story of CAR T-cell therapy in the US
The United States, a leading market when it comes to medical and pharmaceutical trials as well as novel approaches, has so far seen the approval of five CAR T-cell therapies. Because the FDA’s rulings are of significance globally, we can expect to see CAR T-cell therapies approved and applied more widely.
This is good news for patients suffering from cancer/lymphoma who do not respond to traditional therapies: CAR T-cell therapy is a new type of immunotherapy that utilizes a patient’s own, genetically modified, T-cells to find and kill cancer. However, while first patients without remission proved to show that the CAR T-cell therapy is successful, there are still limitations - but with scientists constantly evaluating and improving the status quo, the FDA is set to approve further immunotherapy treatments.
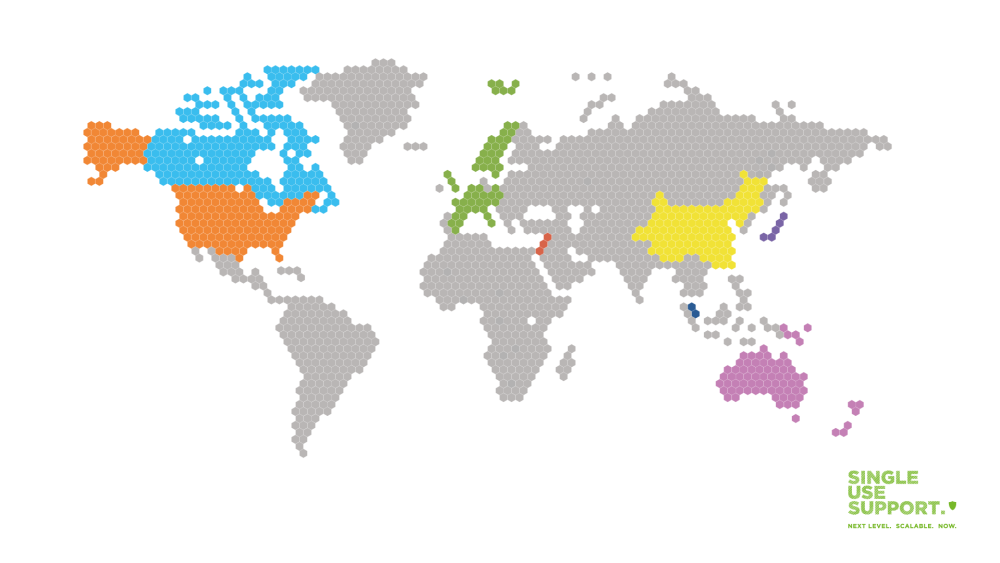
Geographical distribution of the CAR T-cell therapy
So far, the USA and China are leading in terms of CAR T-cell therapy trials and application, with several countries in Europe being in hot pursuit. The geographical distribution for Europe is not evenly spread out but rather shows a concentration in specific regions.

Europe
Spain is the first country with an approval for a CAR T-cell therapy developed entirely in Europe. Using a monoclonal antibody created at the Hospital Clínic de Barcelona more than 30 years ago, it is approved for patients older than 25 suffering from acute lymphoblastic leukemia (a type of blood and bone marrow cancer), with approval for other European countries pending.
Australia
In Australia, CAR T-cell therapy has become available as treatment for patients with certain types of lymphoma as of August 2021. It is expected that up to 300 Australians per year will benefit from access to this novel kind of therapy, which is available at selected public hospitals nationwide.
China
Yescarta, the same type of chimeric antigen receptor cell therapy that has been approved in Australia, is also available in China. In China, the therapy, which was brought to market by US firm Gilead, is expected to cost over RMB 1 million (equalling approx. USD 154,000) per course.
United Kingdom
In the UK, there are currently three types of CAR T-cell therapy used for treatment for select patients: Kymriah, Yescarta and Tecartus have all been approved as immunotherapy treatment for patients suffering from B-cell acute lymphoblastic leukaemia as well as certain types of lymphoma.
Singapore
Singapore has approved its first commercial CAR-T cell therapy with Kymriah in 2021. The approval applies two different cancers: to B-cell acute lymphoblastic leukemia in children and young adults who are between 2 and 25 years old and to refractory diffuse large B-cell lymphoma in adult patients. Singapore General Hospital is known to be the first treatment center in Southeast Asia who offers Kymriah therapy.
Limitations for the roll-out in further countries
While to date there have been around 10,000 patients worldwide to have received CAR T-cell therapy in a clinical trial, there were only a handful of recipients from middle- and low-income countries, with Africa, South America, and India not having registered a single trial. This may come as no surprise, seeing the costs and logistics involved: According to an article published by The Lancet [doi: 10.1016/s2352-3026(21)00068-5], the costs currently stand at between USD 373,000 and 475,000 - per dose.
But costs and logistics are not the only challenges, with scalability being another factor adding to the limitations, irrespective of whether we are speaking of industrialized nations or low-income countries.
This is why Single Use Support have set out to tackle these challenges and offer a solution for logistical challenges: Their end-to-end solutions in CGT allow for a flexible and scalable supply-chain process with solutions throughout the clinical phase, manufacturing upstream, manufacturing downstream and intermediate transport requirements. Such a process is ideal when it comes to elaborate treatments like the CAR T-therapy.