End-to-end solution
March 13, 2023
Safe handling of bulk drug substances
When dealing with bulk drug substances, there are several risks to consider along the processing steps. Read more on the critical steps in bulk drug substance production and possible solutions – in this article.
March 1, 2023
Meeting cGMP regulations with innovative single-use technologies
Manufacturers in the pharmaceutical industry are required to comply with cGMP (Current Good Manufacturing Practice). End-to-end solutions based on automated single-use technologies are an auspicious choice for drug substance manufacturers, given their many advantages. Their implementation in the manufacturing process promises efficiency, high product quality, cost reduction, and energy savings.
March 1, 2023
cGMP - everything you need to know
The current Good Manufacturing Practice (cGMP) regulations - originated and checked by the FDA - apply to companies operating in pharmaceutical, biotech, or med tech industries. They require the latest standards in terms of production, manufacturing, and packaging and thereby ensure pharmaceutical quality meaning quality in manufacturing processes and patient safety as the ultimate goal. The detailed requirements, importance of cGMP, and how companies can best meet the cGMP regulations, are discussed in the following.
February 28, 2023
Reducing product loss & human error in pharmaceutical fermentation
Pharmaceutical manufacturing processes, including pharmaceutical fermentation, are still prone to human error and product loss. The challenges can be overcome by moving to automated processes that eliminate the need for slow and error-prone manual handling. Adopting automated end-to-end solutions based on single-use technologies minimizes, if not eliminates, human error and product loss.
February 10, 2023
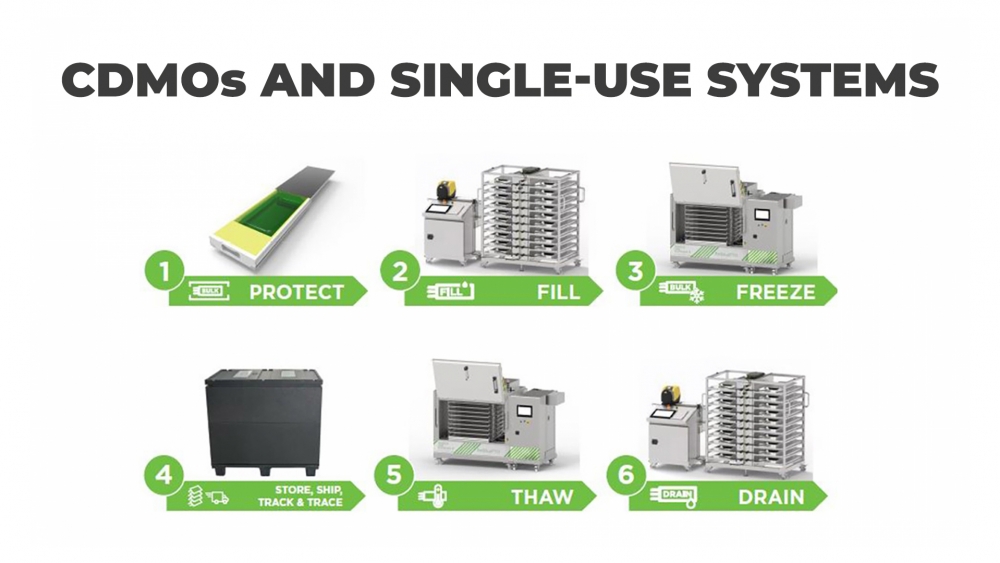
Why CDMOs are increasingly using single-use systems
By combining a number of benefits, single-use technologies that are compliant with cGMP and GAMP seem the perfect choice. For manufacturers of drug substances, they provide a more efficient, cost-effective, and scalable solution than stainless-steel tanks by offering.
February 10, 2023
How to choose a CDMO? 7 Considerations to be made
Choosing the right CDMO or CMO to work with is an important decision. Irrespective of which stage biopharma and pharma companies find themselves in, there are certain things to consider that are valid for any stage throughout your production process.
February 10, 2023
CDMO in Biopharma: Opportunity or risk?
Both, CDMOs and CMOs, are offering major opportunities in biopharma for startups and emerging companies as well as for established players who are approaching the limits of their manufacturing facilities or are generally looking to outsource parts of or their entire commercial production.
December 19, 2022
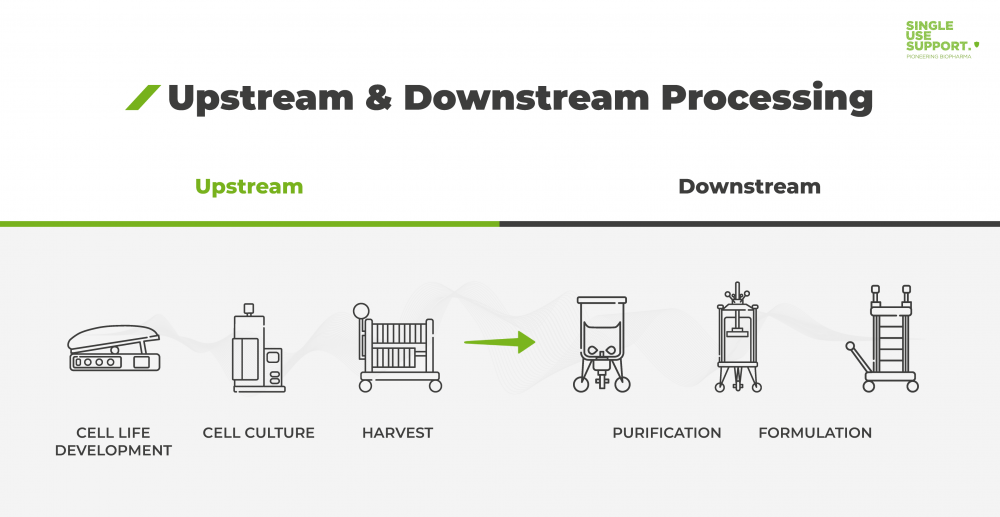
What is upstream and downstream processing?
Upstream and downstream processing are two steps inherent to the production of active pharmaceutical ingredients (API) used in biopharmaceuticals. The production is challenged by increasing regulation and manufacturing costs. This calls for flexible solutions, which is why a growing number of manufacturing processes utilize single-use systems
March 24, 2022
Storage Density in Biopharma Manufacturing Facilities
Single-use bags with its flat shape is predestined for stacking and offer great solutions to increase storage density on-site but also for shipment. The combination of both, single use bag and RoSS® shells (Robust Storage and Shipping) makes them a cost-effective solution, as they require little storage space. RoSS® shells are available for all sizes and suppliers of single-use bags.
March 21, 2022
Scalability of single-use drug substance production
The aim was to achieve a freezing process where the last point of freeze always happens at the same time throughout all scales. If successful, the generated freezing curves will look very similar. Temperature ramps have been used to slow down or accelerate the freezing process.
February 15, 2022
Whitepaper: How controlled freezing enables scalability
In a comprehensive study the impact of ice front growth speed on scalability of freezing protein solutions has been evaluated.
February 2, 2022
Inline buffer dilution: an agile system for downstream processes
Inline buffer dilution (IBD) is a method for on demand buffer preparation from buffer concentrates at the point of use. This article will give an overview of the inline buffer dilution process and discuss its benefits over the traditional formulation of buffer solutions.