February 27, 2020
Case Study: Drug Substance Management with Single-Use vs. Stainless Steel
With bulk drug substances playing an increasingly important role in the pharmaceutical and biopharmaceutical industry, the need for reliable as well as controlled freeze-thaw processes is becoming ever more important.
Not only fast freezing rates but also the protected and safe storage and shipping of bulk drug substances without any risk of contamination are imperative in today’s market.
This is why Single Use Support has conducted a case study to compare the advantages respectively disadvantages of single-use systems and cryovessels. And while stainless steel cryovessels may seem the more logical choice due to sustainability, the study shows that single-use is at least one step ahead by all acounts.
Comparison of the freeze/thaw processes with single-use and stainless steel
Many companies in Biopharma face challenges in the transport of liquid drug substances from the drug substance site, after Downstream process, to the drug product site, before Fill & Finish.
There are many obstacles to overcome in order to preserve the highest possible quality and minimize product damage during this process. However, in order to choose the right technology, also financial aspects play a decisive role.
The aim of this case study is to compare the freezing/thawing process of BDS between both technologies, single-use and stainless steel.

Primary investing – what biopharma manufacturers need to consider
In terms of the total costs of ownership, one needs to distinguish the CAPEX from the OPEX. While the former (capital expenditure) describes the expenses before a system can be implemented, thus mainly the costs for technological solutions and their respective equipment, the latter (operational expenditure) is more focussed on the running costs, such as maintenance, equipment that needs to be replaced, but also the expenses on personnel. Both aspects are vital to be considered before the decision, whether single-use or stainless-steel systems shall be implemented, can be made.
CAPEX – Capital expenditure of single-use vs. stainless-steel technologies
While both single-use and stainless-steel facilities require two freeze/thaw units to be implemented, the equipment for the latter is far more expensive. This is due to the several tanks and cryo-units that, compared to single-use products, are much more cost-intensive.
OPEX – Operational expenditure of single-use vs. stainless-steel technologies
Regarding the ongoing costs for the operation of single-use technologies, these are lowered by the fact that the respective components are designed for one single usage. As a consequence and in contrast to stainless-steel vessels, single-use products do not need to be elaborately cleaned, which saves both costs and resources, as this lowers energy and water consumption. This also means that single-use technologies can be operated by smaller teams.
An additional factor that lowers the OPEX when using single-use technologies is the reduced risk of contamination; as they come pre-sterilized and do not need to be cleaned after usage, this risk is practically eliminated – considering how valuable the substances are that biopharma manufacturers deal with, this is a circumstance that is not to be neglected.
Last but not least, single-use solutions need less storage space and – as they are not to be sent back after usage – reduce transport costs.

Single-use technologies – the right choice?
The answer to this question remains to be determined by the respective manufacturing site and its specialization, thus cannot be answered in general. Despite the factors described above, there are other considerations that need to be taken into account, apart from costs alone, that might make it easier for stakeholders to make a decision.
Speaking of the case study, however, it was shown that the implementation of single-use technologies allows BDS to save up to 3,000,000 Euro after two years.
Given the huge amount of advantages that single-use solutions can bring to biopharma manufacturing sites, it is not surprising that the industry is in great need for specialized, reliable solutions.
Single Use Support have set the goal to provide the biopharmaceutical industry with solutions that support them in increasing the reliability of storage and shipping, in turn minimizing the risk of contamination and the loss of valuable high-quality drug substance.
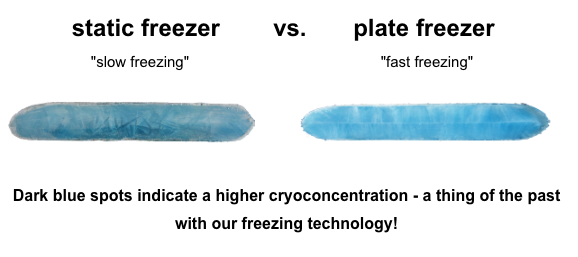
The freezing of liquid solutions is a complex process that poses its own challenges and may lead to Cryoconcentration or the formation of aggregates followed by denaturation. Both of these scenarios are less than ideal: They will invariably lead to the loss of valuable and potentially life-saving drug substance.
High quality freezing is important for a reliable cold chain process

This is where Single Use Support comes in with its platform for safe and reliable freezing and thawing for all stages of the production process – from lab to blockbuster.
By conducting extensive research and a multitude of tests, Single Use Support has come to the conclusion that a combination of plate freezers and single-use bags offer the ideal solution for a safe and reliable freezing process of protein solutions and bulk drug substances.
This seemingly simply combination of two components – plate freezer and single-use bioprocess container – separates the protein solution from the cold surface by only a thin polyethylene layer, thus ensuring an optimal heat transfer. It also allows for a faster freezing front velocity and improved temperature control when compared to plastic bottles or multi-use stainless-steel cryovessels.
Single Use Support has developed a plate freezing solution that can handle volumes of up to 300l contained in 50l single-use bags. The bags are encased in RoSS@ shells that are constructed of a plastic frame with the base and lid made of stainless steel. This combination of materials not only offers added protection but also facilitates optimal heat transfer rates during the thawing process.
And last but not least: With its fast freezing properties, Single use Support's solution covers the entire process in less than 2 minutes. It is scalable and offers you a fully reliable and controlled freezing process of bulk drug substances.




