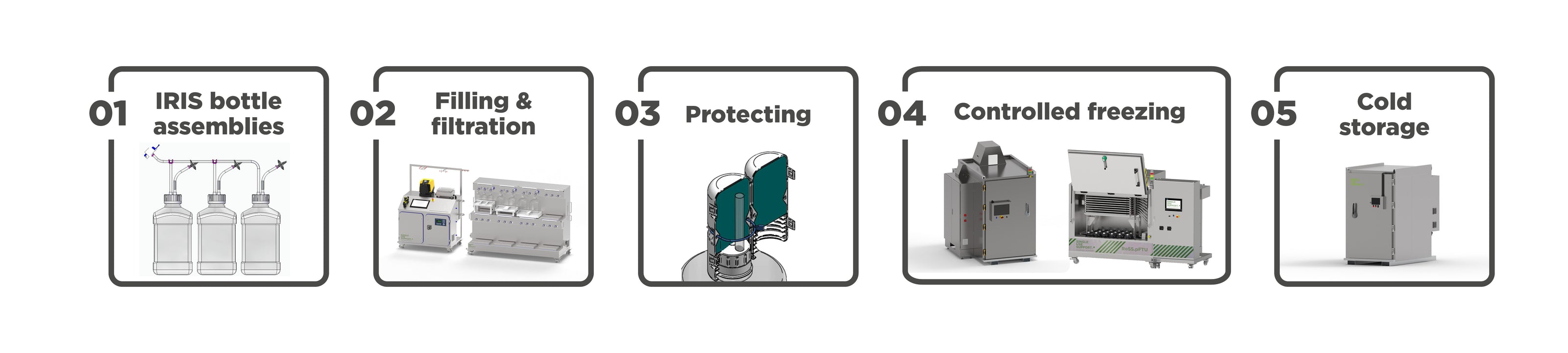
Products
Downloads

Solution overview: Bulk drug substances
Please fill out the form below to download this file.

Solution overview: Bulk drug substances

App Note: The Chilled Future of RNA Therapeutics Filling & Freezing Applications
Please fill out the form below to download this file.

App Note: The Chilled Future of RNA Therapeutics Filling & Freezing Applications

Study: Scalable freezing of protein-based biologics
Please fill out the form below to download this file.

Study: Scalable freezing of protein-based biologics