GENE THERAPY

Gene Therapy Manufacturing Solutions
Gene therapy – modifications on a patient’s genome, e.g. by integration and activation of new genetic material or the deactivation of disease-causing or faulty genes via a viral carrier – have allowed to set milestones in the therapy of a variety of even rare diseases. But although being applied in a more and more experienced manner also beyond clinical trials, genetic therapies will keep requiring the highest possible diligence.
Single Use Support GmbH provides biotechnology companies with specialized single-use solutions for gene therapies to optimize ATMPs (advanced therapies) manufacturing processes – from sterile single-use systems and customizable liquid management systems up to freezing and storing solutions.
Primary packaging for gene therapies
After gene therapy products such as viral vectors have undergone a gene editing process and have been cultured, they are not usually administered immediately. Instead, they must be safely stored ex vivo before they can perform the genetic modification for which they were designed, such as the replacement of a mutated gene or gene transfer.
To ensure the quality and safety of gene therapy products until they are transported to readministration, Single Use Support has developed single-use solutions in different sizes: For instance, RoSS.KSET is designed for the protection of smaller bioprocess containers, such as those needed for gene therapy, where smaller volumes need to be handled.
Solutions
-
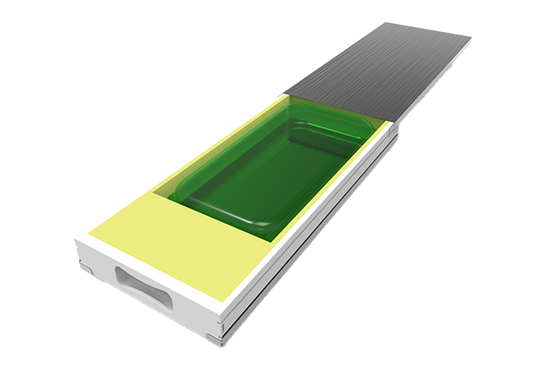
Protection for small single-use bags | ROSS.KSET
RoSS.KSET offers protection for small single-use bags. Most suitable for small batch sizes. Smart protection for single-use bags with volumes less than 250 mL in cell and gene therapy or clinical studies.
Bag independent
Reduced product loss
Best freezing result
-
Protecting single-use bags | RoSS® Shell
The safest transport solution for all available single-use bioprocess containers. Protect your single-use bag and reduce product loss. RoSS®: Robust Storage & Shipping
Bag independent
Reduced product loss
Best freezing result
-
2D bioprocess container | IRIS Bag
Innovative. Robust. Individual. Single-Use. Make sure your supply chain is uninterrupted. Get your IRIS 2D single-use bioprocess container from Single Use Support in any size with the fastest possible delivery times. Store and ship anything from c...
Sizes from 20mL-10L
Freezing to -85°C
Reliable partner
Aseptic Filling and Draining of gene therapy products
To make sure that gene therapy works safely and effectively, the products that patients are provided with must be handled in a secure and sterile manner. This also applies to the filling and draining process, where the risk of cross-contamination and exposure to the environment must be avoided at all costs. Therefore, it is advisable to automate fluid management in gene therapies.
Single Use Support has developed dedicated gene therapy liquid solutions for safe and efficient filling and draining processes – the RoSS.FILL platform is the result of these efforts. The devices are available in different sizes, allow for high flexibility while speeding up turnaround time in gene therapy production.
Solutions
-
Cell and gene therapy filling system | RoSS.FILL CGT
RoSS.FILL CGT is a fully automated aseptic cell filling and viral vector filling system that allows multiple small single use bags to be filled simultaneously. With its high filling accuracy, the system is special designed for use in cell & ge...
Highest accuracy
Down to 1mL/Bag
Integrated sealing & perforation
-
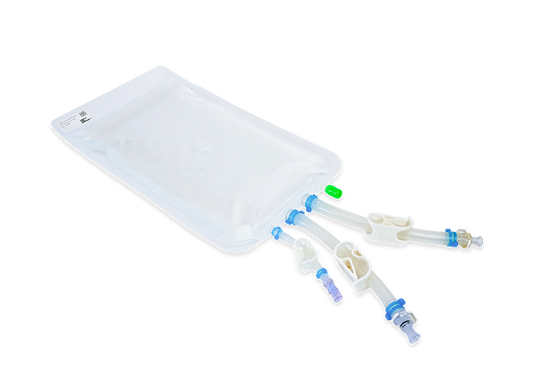
Aseptic filling system | RoSS.FILL Bag
RoSS.FILL Bag is a flexible automated aseptic filling machine for the aliquotation and dispensing of bulk drug substance (BDS) into single-use bags. The system for aseptic filling and sterile filtration is highly precise, making RoSS.FILL Bag an e...
Up to 400L+/Batch
Highest accuracy
Fast throughput
-
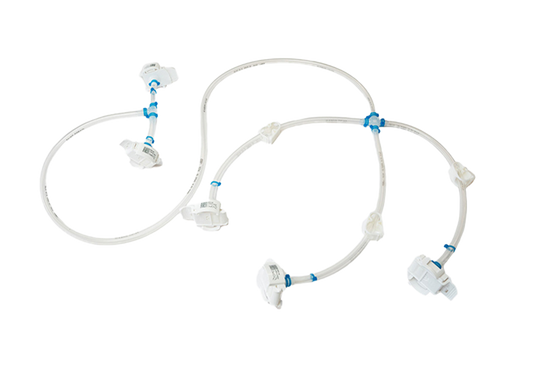
Biopharma fluid transfer | IRIS Single-Use Assemblies
As an expert in single-use solutions, we have made it our goal to deliver vendor agnostic single-use assemblies manufactured at highest quality standards in ISO 7 cleanrooms and sterilized within shortest lead times. Prevent downtime and ensure an...
100% customizable
Dual sourcing
GMP-compatible
Controlled Freeze and Thaw processes for genetic therapies
Gene therapy products have very specific requirements for their freeze/thaw conditions, and an uncontrolled freezing process might cause irreparable damage to the product. Viral vectors, for example, are not only best stored at ultra-cold temperatures around -80 °C, but also need to be brought to this temperature at controlled cooling rates as they, too, are crucial to their quality.
Single Use Support has been innovating in this field and developed the RoSS.pFTU platform for optimized freezing and thawing of drug products. Based on plate freezing techniques, our freezers are available in different sizes, therefore suitable for various fields of application – be it the freezing of small volumes with RoSS.pFTU Lab Scale or the handling of larger volumes with the RoSS.pFTU Large Scale plate freezer, achieving temperatures as low as -80 °C. If even lower temperatures are required, the cryogenic freezer RoSS.LN2F can freeze products down to -150 °C.
Solutions
-
Ultra-low temperature storage freezer | RoSS.ULTF
RoSS.ULTF is an upright ultra-low temperature storage freezer for frozen drug substances in different sizes. The ultra cold storage freezer keeps the desired set point temperature down to -80°C. It is compatible with RoSS® Shells to protect your ...
Down to -80°C
Capacity for up to 300L
Modular Interior
-
Cryogenic freezer | RoSS.LN2F
RoSS.LN2F is a powerful cryogenic controlled rate freezer for cryopreservation down to -170°C. An enclosed and innovative platform system for dosed freezing for cell and gene therapies eliminates direct exposure and the need for mechanical compres...
Increased cell viability
Down to -170°C
Down to < 250mL/ Bag
-
Laboratory freezer | RoSS.pFTU Lab-Scale
Single Use Support's plate-based laboratory freezer is the perfect solution for clinical studies conducted in labs and, above all, if you want to start a controlled and scalable cGMP freezing & thawing process. The system is compatible with si...
Down to -80°C
Controlled freeze-thaw
Up to 10L
Gene therapy products – transport solutions
Gene therapy products have very specific requirements for their freeze/thaw conditions, and an uncontrolled freezing process might cause irreparable damage to the product. Viral vectors, for example, are not only best stored at ultra-cold temperatures around -80 °C, but also need to be brought to this temperature at controlled cooling rates as they, too, are crucial to their quality.
Single Use Support has been innovating in this field and developed the RoSS.pFTU platform for optimized freezing and thawing of drug products. Based on plate freezing techniques, our freezers are available in different sizes, therefore suitable for various fields of application – be it the freezing of small volumes with RoSS.pFTU Lab Scale or the handling of larger volumes with the RoSS.pFTU Large Scale plate freezer, achieving temperatures as low as -80 °C. If even lower temperatures are required, the cryogenic freezer RoSS.LN2F can freeze products down to -150 °C.
Solutions
-
Cold chain container for drug substances | RoSS.SHIP
RoSS.SHIP secures the cold chain storage and shipping of bulk drug substance for almost one week. With its Smart Tracking & Tracing Add-on you never loose control of your product. Highly robust, stackable, coolable and compact – RoSS.SHIP co...
Below -60°C for at least 6 days
Track & trace 24/7
Up to 55 RoSS® shells
-
Stainless steel rack for RoSS® shells | RoSS.RACK
RoSS.RACK is a supporting device for storage and international shipping of up to 30 RoSS® shells. It is available in different sizes as single-use or multi-use rack. The multi-use rack is made out of stainless steel, and therefore suitable for th...
Up to 30 RoSS® shells
Suitable for cleanroom
100% customizable