PUPSIT system | RoSS.PPST
PUPSIT system | RoSS.PPST
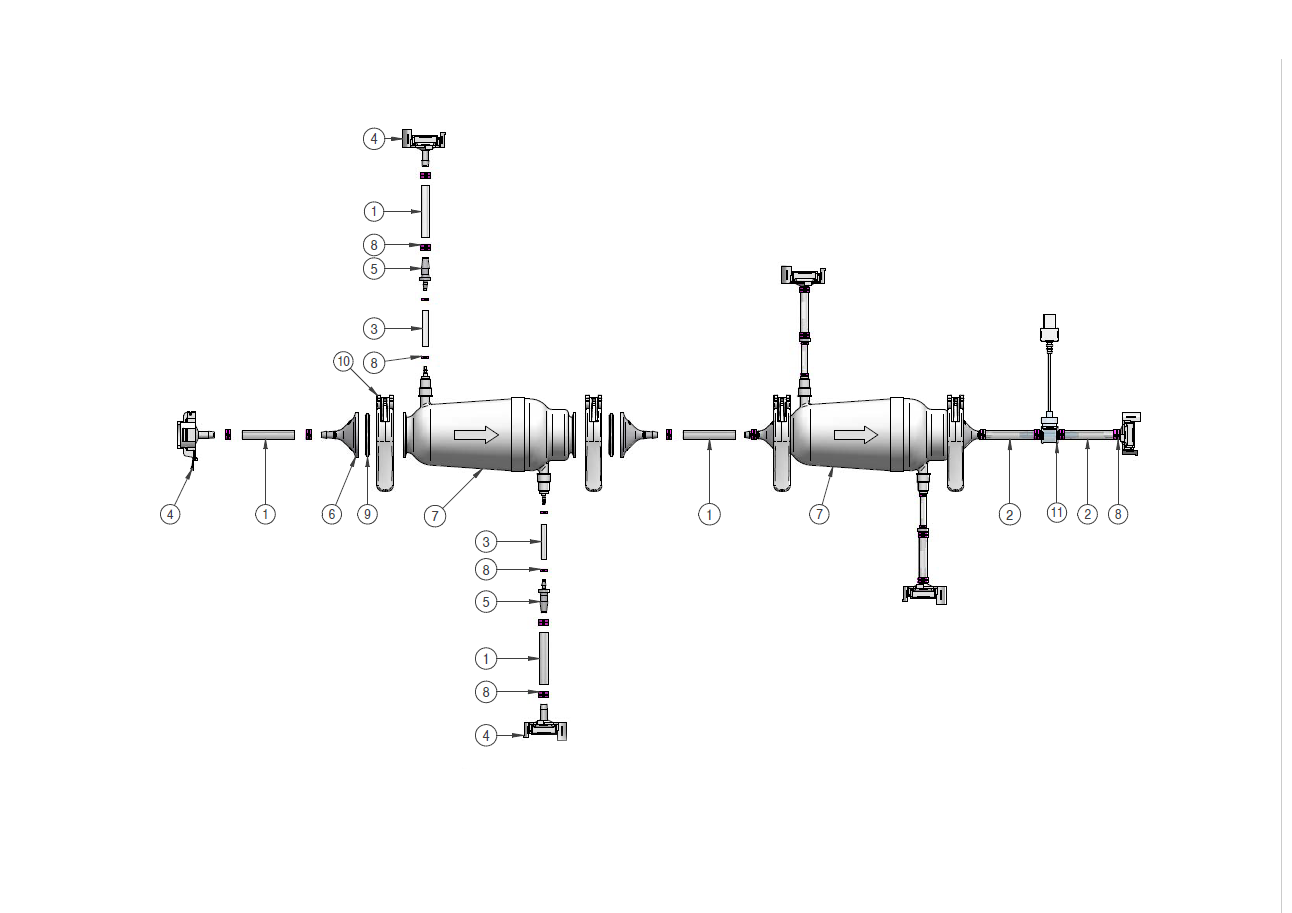
RoSS.PPST is an advanced PUPSIT system that tests the integrity of up to two filters used in biopharmaceutical manufacturing. It verifies filter integrity after sterilisation and prior to aliquotation, meeting EU GMP Annex 1 relevant quality standards. With intuitive operator guidance and real-time monitoring of pressure and flow, RoSS.PPST minimizes the risk of errors and contamination.


Features and benefits of our PUPSIT solution
- Standard configuration: Available as standard version with the option to test two filters
- Compatible with all common product filters
- User-friendly workflow
- Suitable for cleanroom and GMP-compliant construction
- Seamless integration into end-to-end solutions from Single Use Support
- Additional products and services available including single-use assemblies for PUPSIT
What makes RoSS.PPST the optimal PUPSIT system?
-
Customizable setup
Aseptic processes vary - so should your PUPSIT solution. Our fully customizable PUPSIT skid adapts to specific filter integrity testing needs, offering flexible PUPSIT assembly options. We collaborate closely with customers to deliver tailored, process-ready systems.
-
Simple & seamless integration
The PUPSIT rack seamlessly connects with our innovative RoSS.FILL system, enabling a fully automated aliquotation process in sterile manufacturing. Alternatively, RoSS.PPST can operate as a standalone platform.
-
Prevent product loss
Our automated single-use PUPSIT (pre-use post sterilization integrity testing) solution detects filter issues, helping to maintain product quality, prevent contamination, and avoid costly product losses.
-
GMP-compliant
Our sterile filtration system in the pharmaceutical industry is fully compliant with EU GMP Annex 1 and global regulations - designed to prevent microscopic flaws that could risk product quality or patient safety.
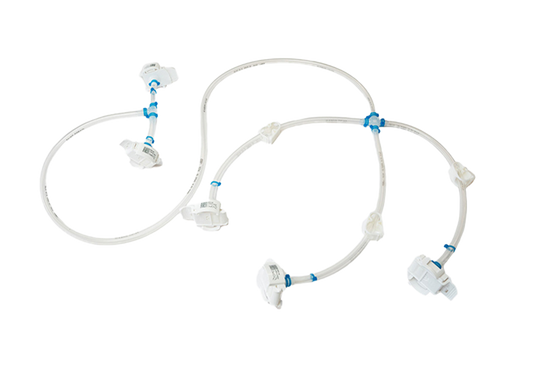
Your filter single-use assemblies
We offer manufacturer-independent single-use PUPSIT assemblies, designed and delivered to meet individual process requirements.
Downloads
More solutions
-
Biopharma fluid transfer | IRIS Single-Use Assemblies
As an expert in single-use solutions, we have made it our goal to deliver vendor agnostic single-use assemblies manufactured at highest quality standards in ISO 7 cleanrooms and sterilized within shortest lead times. Prevent downtime and ensure an...
100% customizable
Dual sourcing
GMP-compatible
-
Homogenizing and cooling system | RoSS.PADL
RoSS.PADL is a scalable massaging and mixing system for achieving a uniform mixture in single-use bags. With integrated cooling and heating, it maintains optimal temperatures consistently. This makes it well suited for applications such as cell &a...
Bag independent
Consistent homogeneity
Temperature control
-
Aseptic cell filling system | RoSS.FILL CGT
RoSS.FILL CGT is a fully automated aseptic cell filling system that allows multiple small single use bags to be filled simultaneously. With its high filling accuracy, the system is special designed for use in cell & gene therapies for up to 12...
Highest accuracy
Down to 1mL/Bag
Integrated sealing & perforation
-
Aseptic filling system | RoSS.FILL Bag
RoSS.FILL Bag is a flexible automated aseptic filling machine for the aliquotation and dispensing of bulk drug substance (BDS) into single-use bags. The system for aseptic filling and sterile filtration is highly precise, making RoSS.FILL Bag an e...
Up to 400L+/Batch
Highest accuracy
Fast throughput