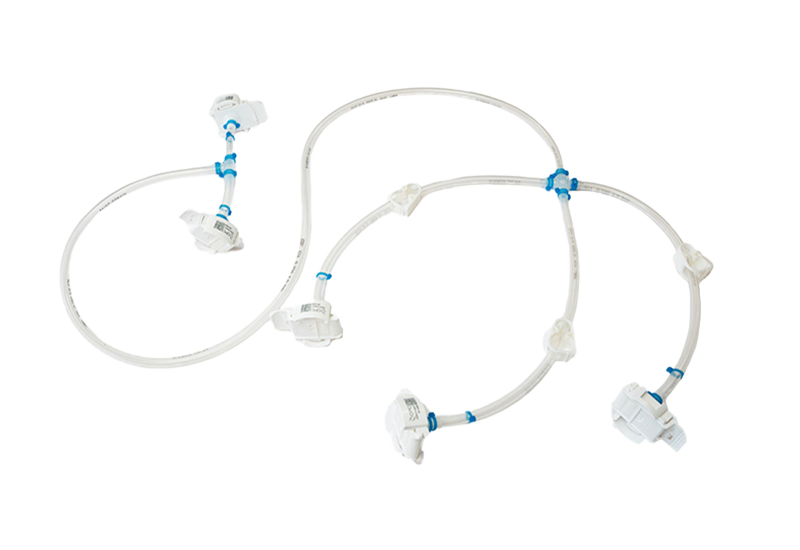
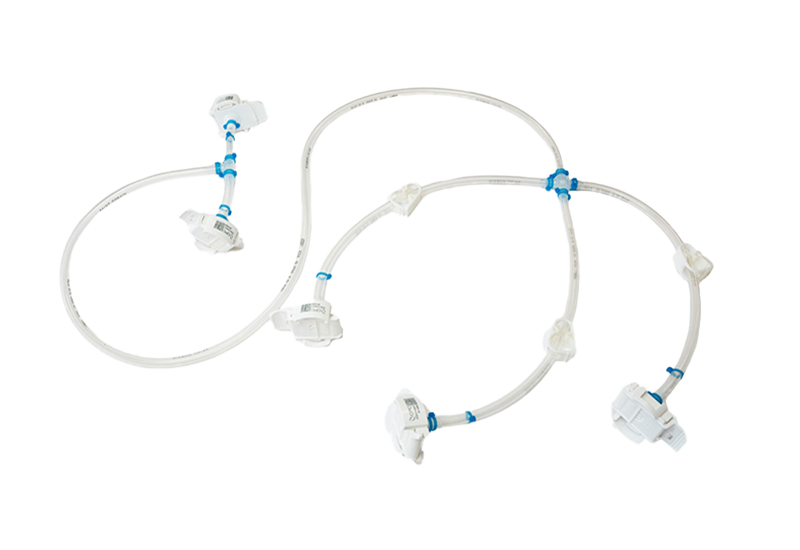
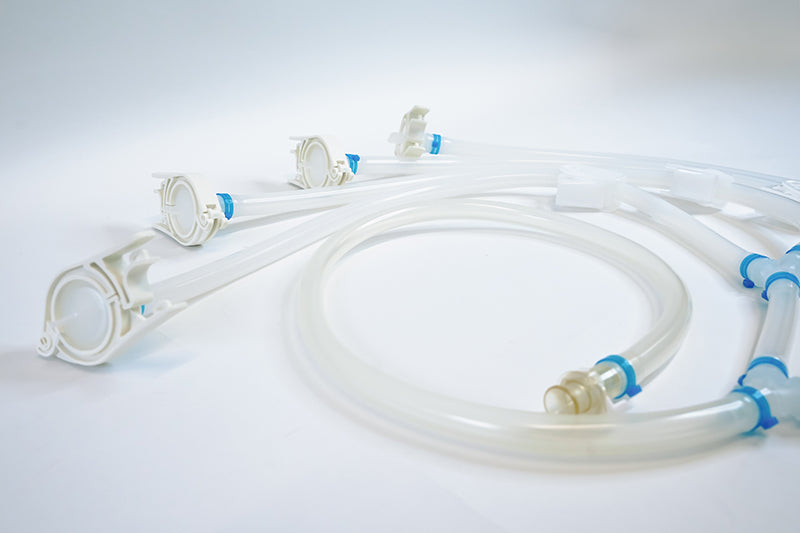
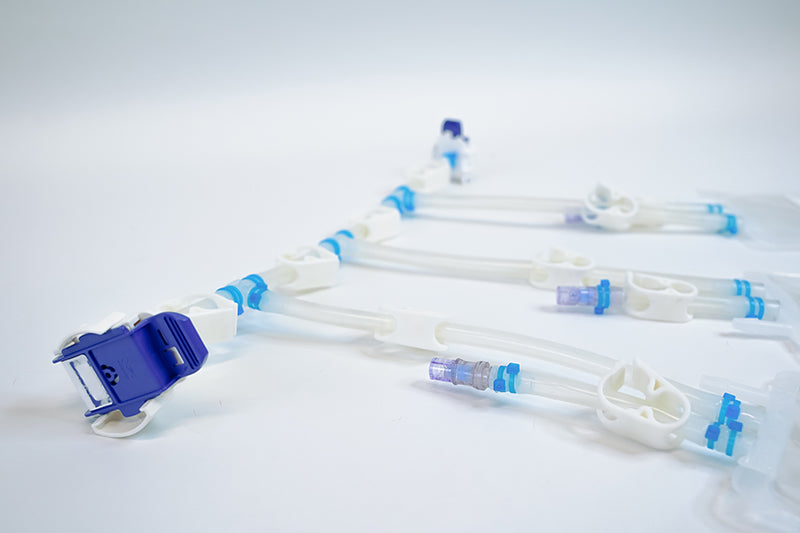
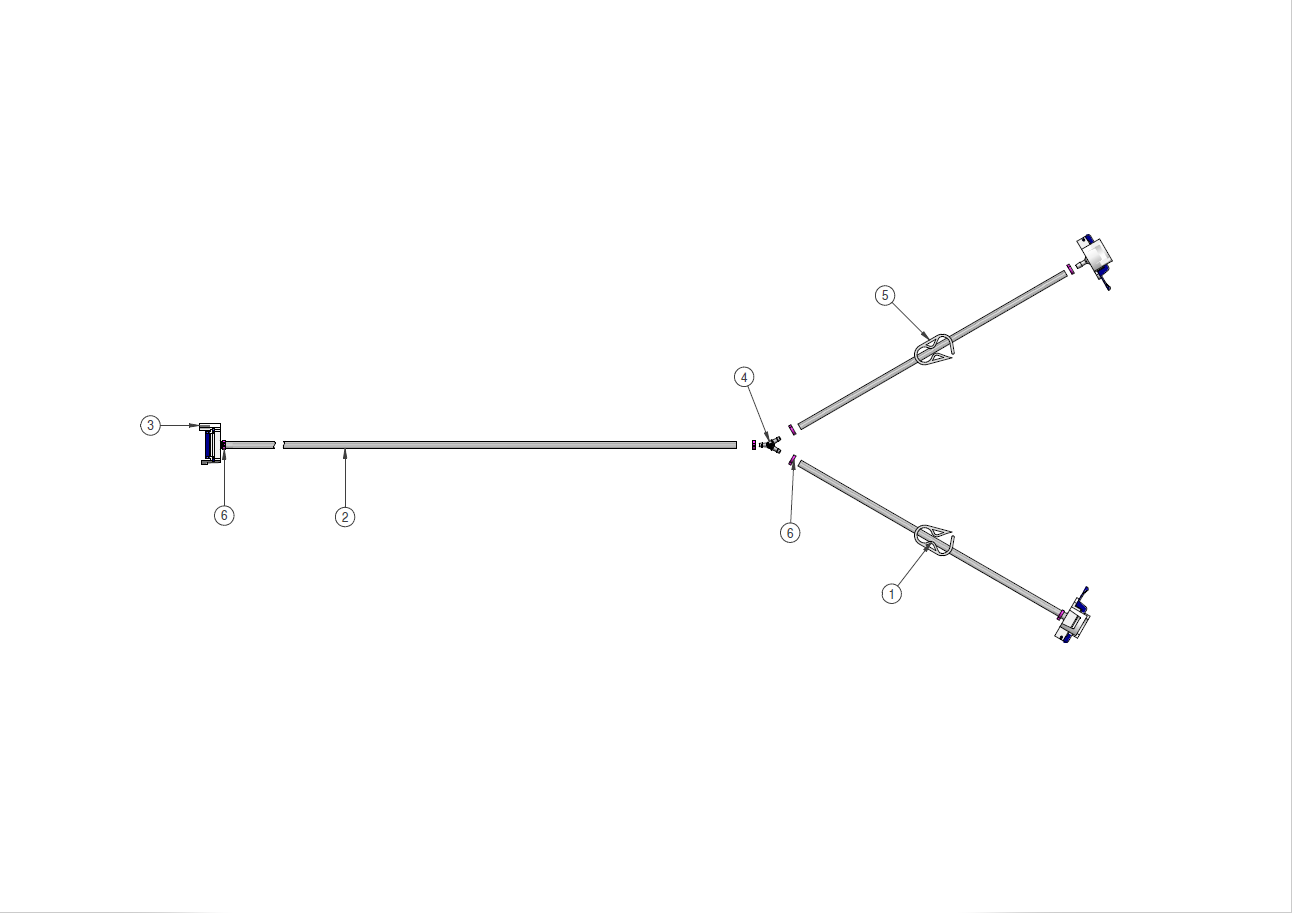
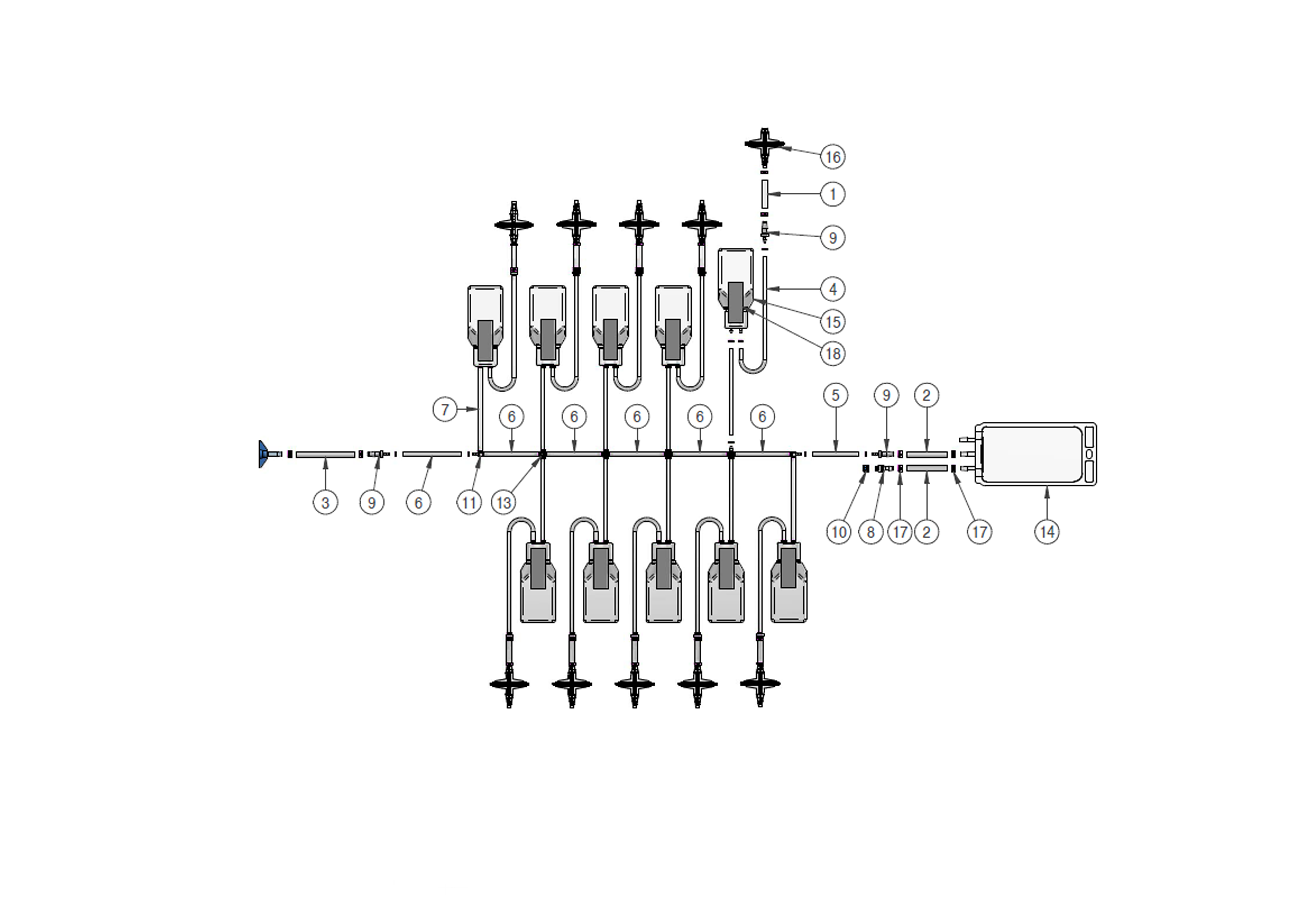
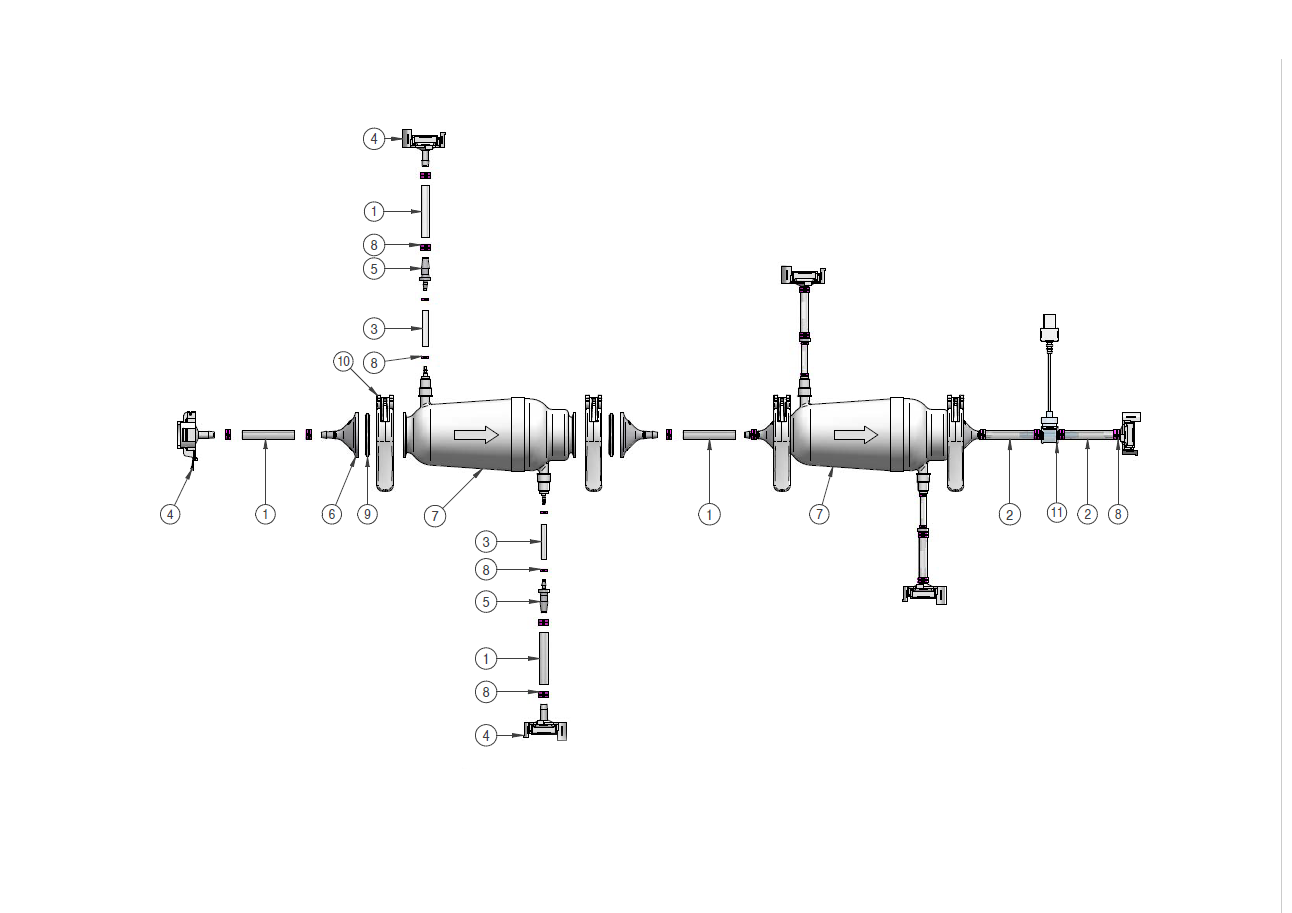
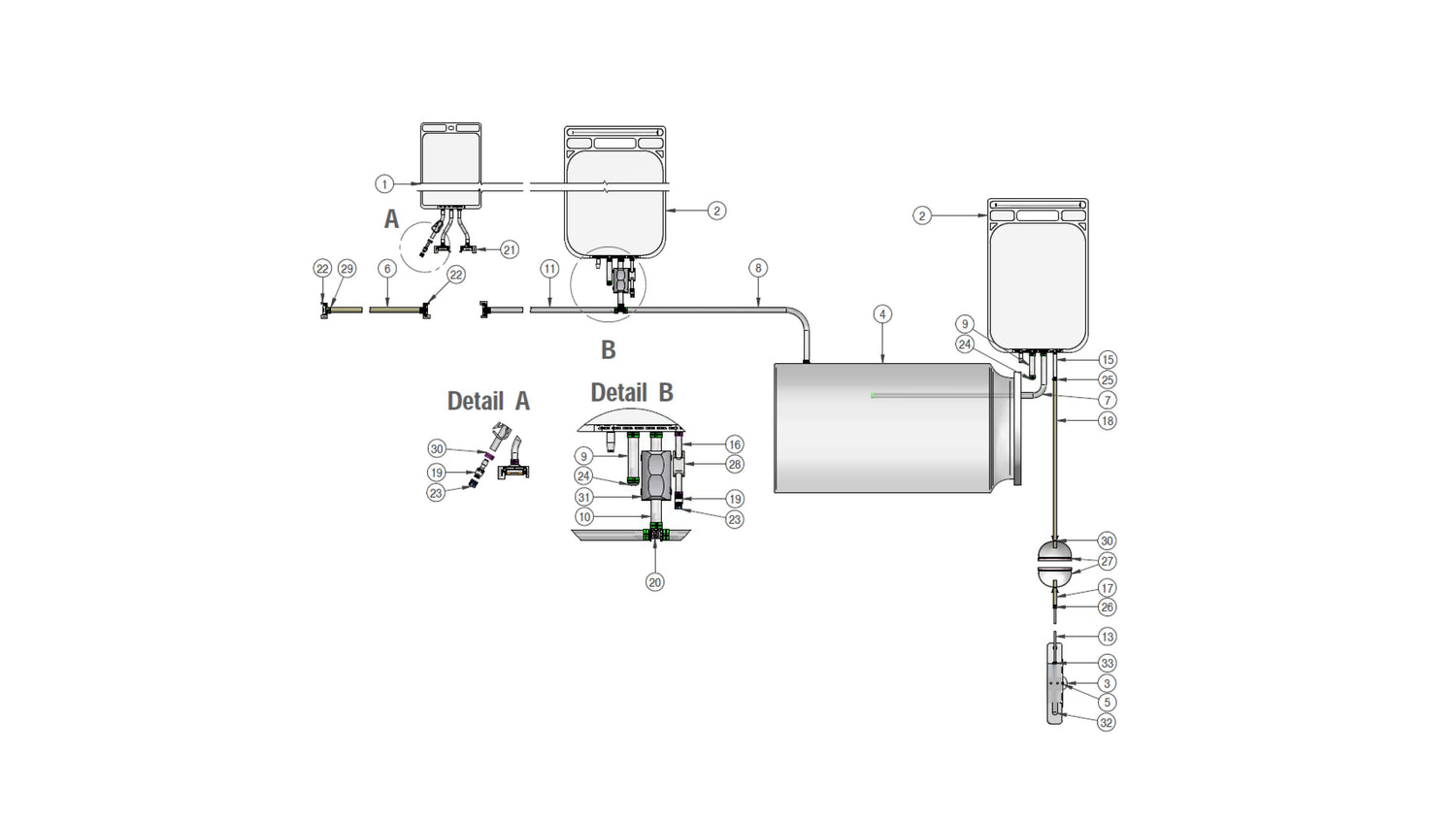
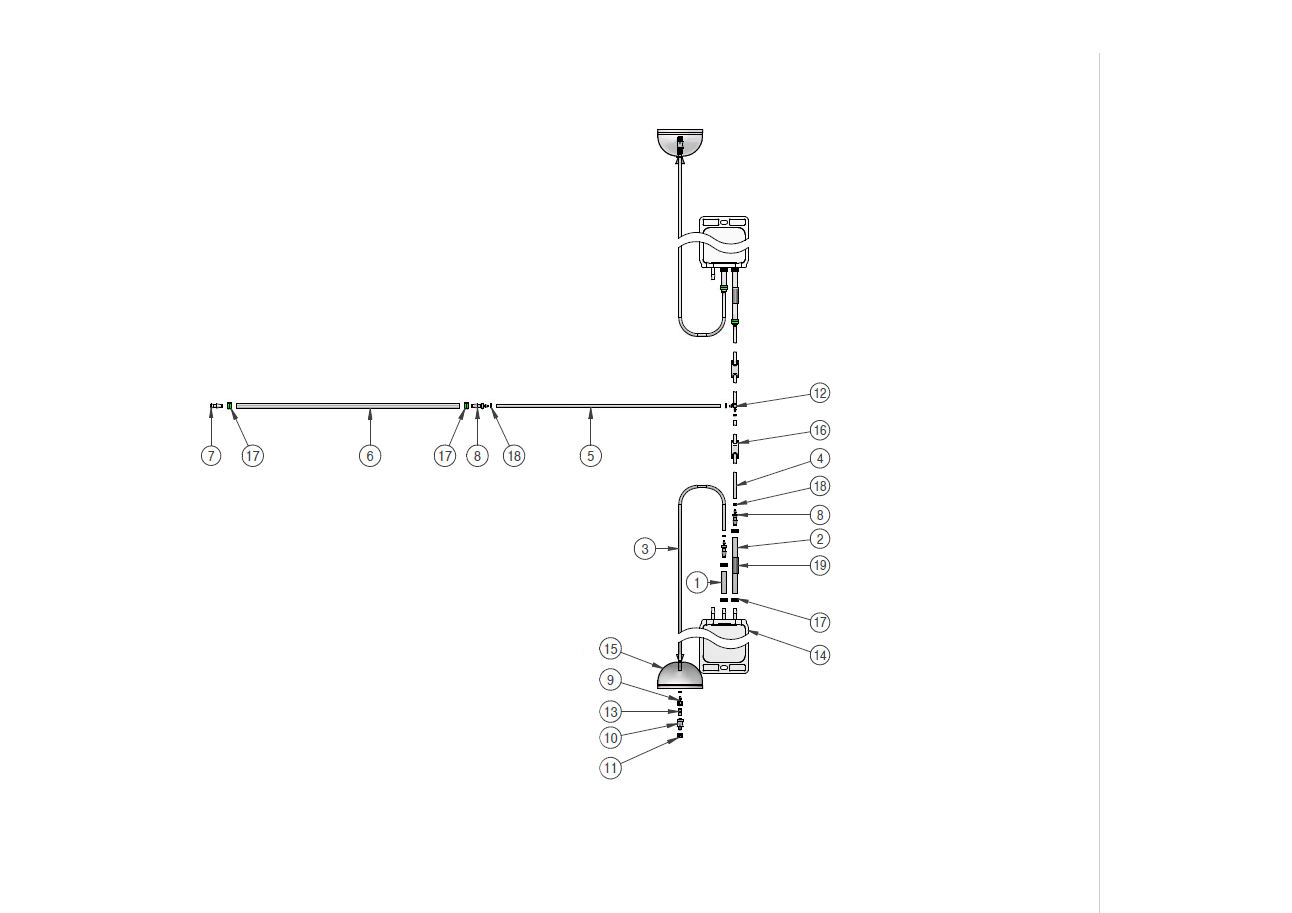
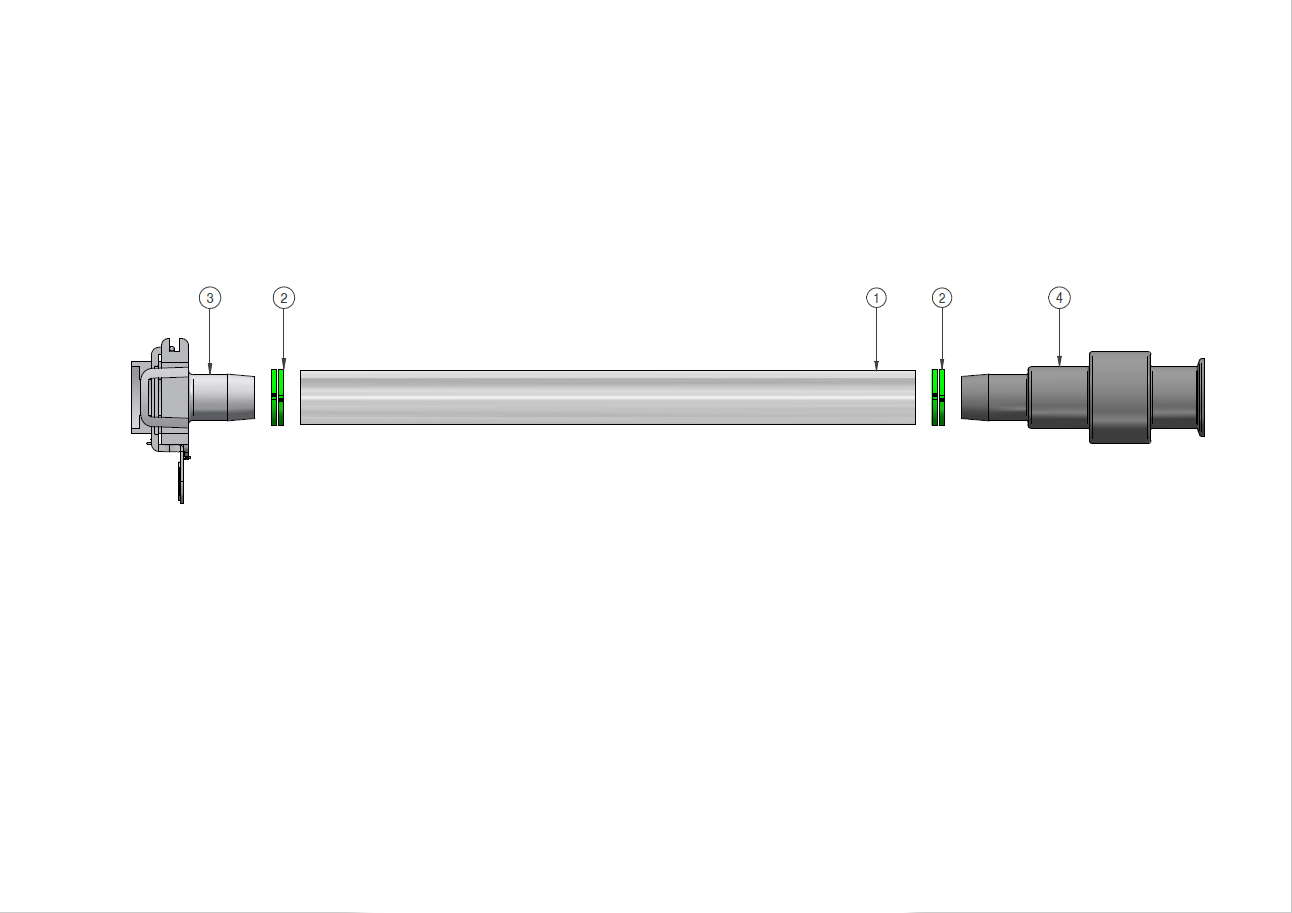
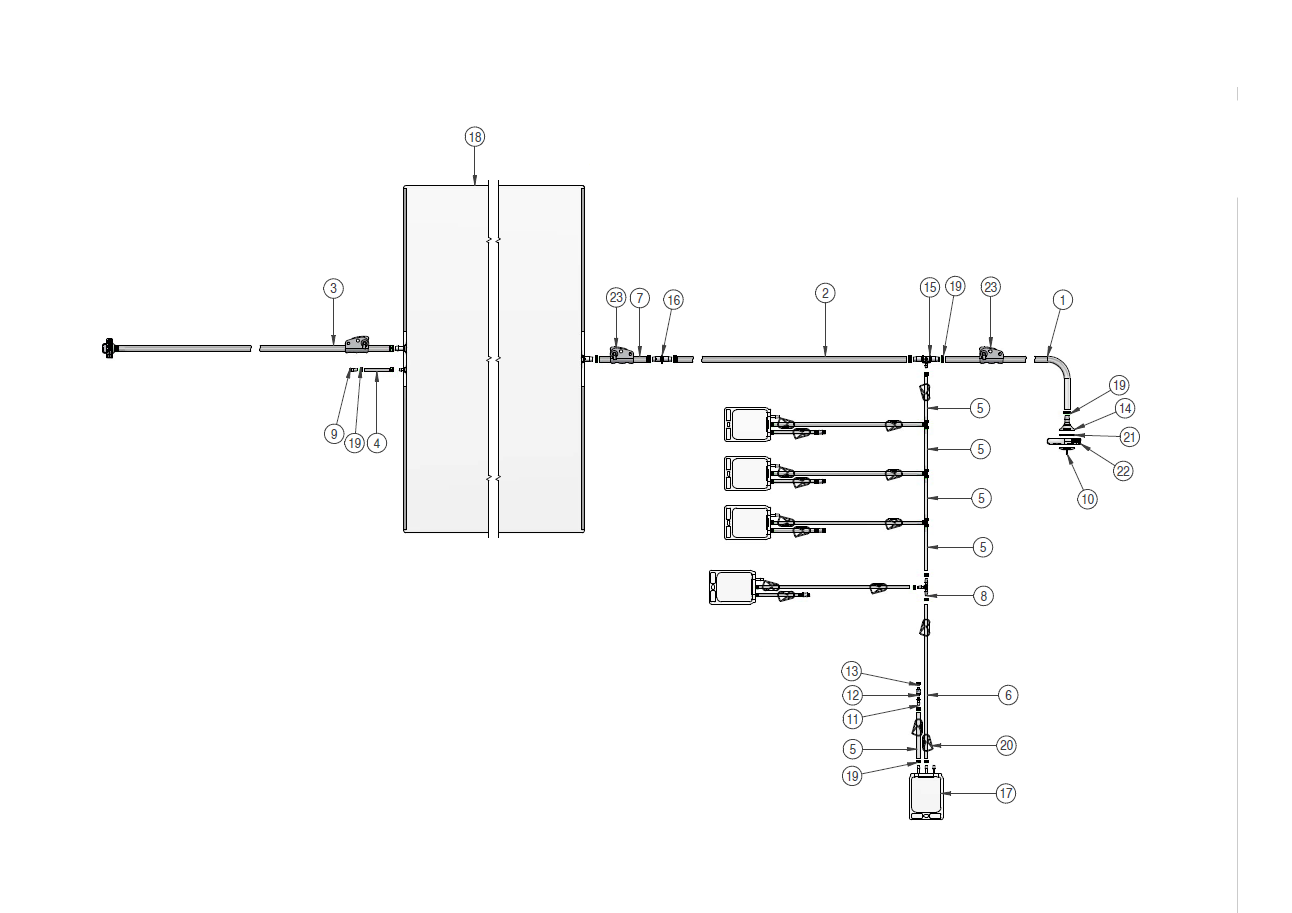
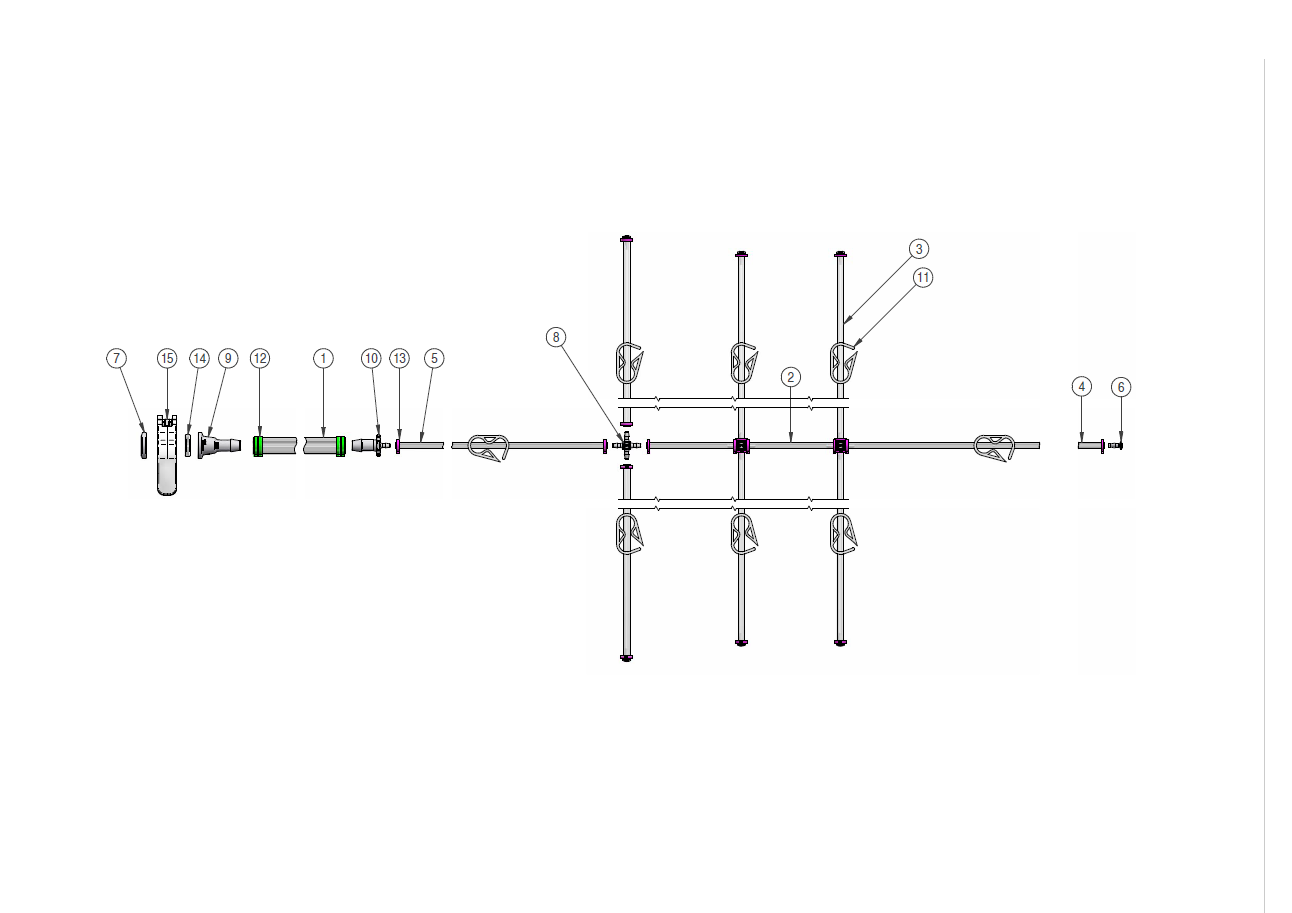
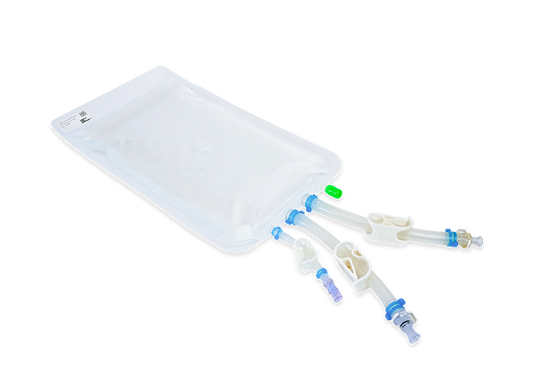
Components used for IRIS Single-Use Assemblies
Single-use assembly catalogue
Downloads

Datasheet: IRIS Single-Use Assemblies
Please fill out the form below to download this file.

Datasheet: IRIS Single-Use Assemblies

Case study: IRIS Single-Use Assemblies Capabilities
Please fill out the form below to download this file.

Case study: IRIS Single-Use Assemblies Capabilities

Whitepaper: IRIS Single-Use Assemblies - Connecting the dots
Please fill out the form below to download this file.

Whitepaper: IRIS Single-Use Assemblies - Connecting the dots