Future-proof your biologics cold chain
Crying over product loss after freezing?
Protein instability after freezing can ruin an entire batch of biologics. Controlled freezing of drug substances in single-use bags and bottles helps manufacturers maintain product quality and increase productivity.
Challenges in biopharma cold chain management
- Product quality: Uncontrolled freezing can compromise critical quality attributes of drug substances, leading to instability, cryoconcentration, and loss of product integrity.
- Compliance: Without validated & documented freeze–thaw processes, GMP‑aligned contamination control and data integrity requirements are at risk of non‑compliance.
- Scalability: Biologics manufacturers face complexities when scaling from clinical phases to commercial production without compromising product quality.

Why choose Single Use Support?
- Minimized product loss: integrity of drug substances throughout the entire cold chain
- Vendor-agnostic: independent from type and brand of single-use bags and bottles
- Scalable: scale-up and -out while maintaining maximum product quality
End-to-end solutions for cold chain processes
-
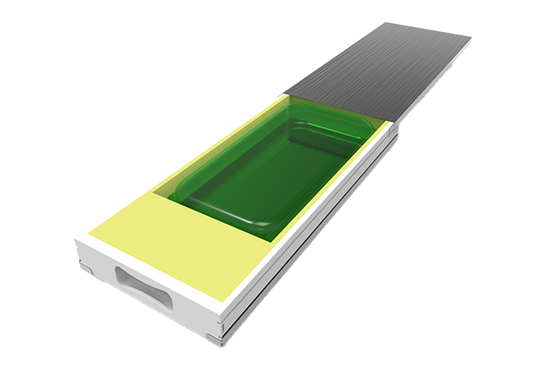
Protecting single-use bags | RoSS® Shell
The safest transport solution for all available single-use bioprocess containers. Protect your single-use bag and reduce product loss. RoSS®: Robust Storage & Shipping
Bag independent
Reduced product loss
Best freezing result
-
Plate freezer biopharma | RoSS.pFTU Large-Scale
Single Use Support's large scale system is a plate-based freeze-thaw unit for any scale and batch size. The system is compatible with single-use bags of all sizes and manufacturers and enables a controlled process down to -80°C of up to 400L per b...
Down to -80°C
Controlled freeze-thaw
Up to 400L+/Batch
-
Blast freezer for pharmaceuticals | RoSS.BLST
RoSS.BLST is a GMP-compatible system for blast freezing & thawing of drug substances. It is suitable for any primary packaging from all manufacturers and allows freezing and thawing within a temperature range of -80°C to +40°C. The modular and...
Capacity for up to 300L
Modular Interior
Down to -80°C
-
Ultra-low temperature storage freezer | RoSS.ULTF
RoSS.ULTF is an upright ultra-low temperature storage freezer for frozen drug substances in different sizes. The ultra cold storage freezer keeps the desired set point temperature down to -80°C. It is compatible with RoSS® Shells to protect your ...
Down to -80°C
Capacity for up to 300L
Modular Interior
-
Cold chain container for drug substances | RoSS.SHIP
RoSS.SHIP secures the cold chain storage and shipping of bulk drug substance for almost one week. With its Smart Tracking & Tracing Add-on you never loose control of your product. Highly robust, stackable, coolable and compact – RoSS.SHIP co...
Below -60°C for at least 6 days
Track & trace 24/7
Up to 55 RoSS® shells
Controlled and scalable freezing of biologics

Download App Note
App Note: Cold Chain Integrity for Biologics
What impact does controlled end-to-end cold chain management have on preserving the quality, potency and safety of biologics? Find out how Single Use Support's modular, automated technologies for single-use bags and bottles minimize cryoconcentration, prevent container damage and ensure consistent, sustainable handling that aligns with Annex 1 throughout bioprocessing.