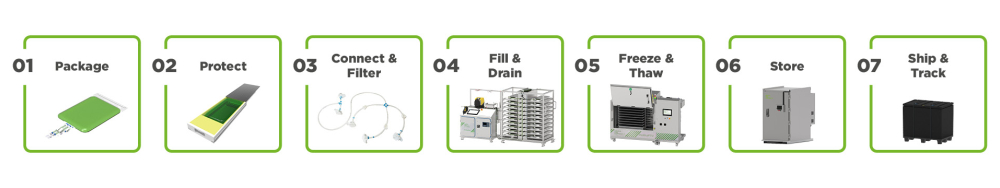
Advanced Fluid Management & Cold Chain for Small Volumes
The single-use technologies based on RoSS® are a combination of integrated platform systems, sterile consumables and auxiliary services to achieve process excellence in commercial GMP handling of small volumes down to 1mL.