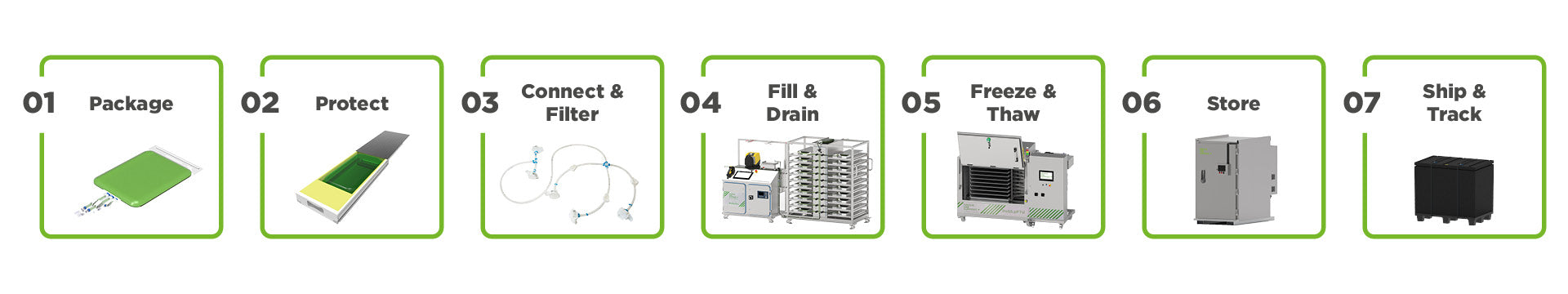
Safe and reliable end-to-end logistic solutions
Downloads

Solution overview: Bulk drug substances
Please fill out the form below to download this file.

Solution overview: Bulk drug substances

Solution Overview Small Volumes
Please fill out the form below to download this file.

Solution Overview Small Volumes