FILL/FILTRATION-FREEZE/THAW
Advanced Fluid Management and Freeze/Thaw Logistics
Automated processes for aseptic fluid management and controlled cold chain management optimize manufacturing of drug substance to a large extent. Different drug substances have one necessity in common: control and reproducibility. cGMP-compliant single-use technologies enable standardization in critical process steps where a large technology gap exists in biopharmaceutical production.
Reduced product loss when handling single-use bioprocess containers, highly accurate and automated filtration and aliquotation and controlled freezing and thawing improve bioprocess steps for all modalities; increased cost-effectiveness, higher drug product quality, rapid performance and limitless scalability.
Primary & Secondary Packaging
-
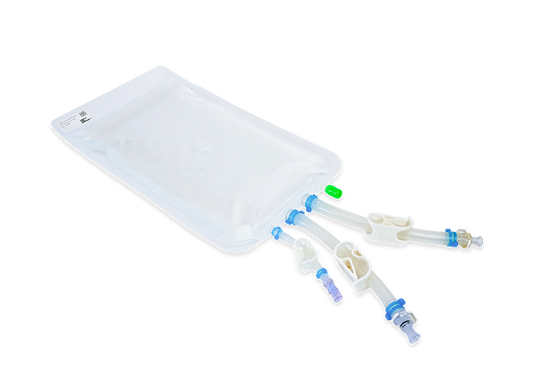
2D bioprocess container | IRIS Bag
Innovative. Robust. Individual. Single-Use. Make sure your supply chain is uninterrupted. Get your IRIS 2D single-use bioprocess container from Single Use Support in any size with the fastest possible delivery times. Store and ship anything from c...
Sizes from 20mL-10L
Freezing to -85°C
Reliable partner
-
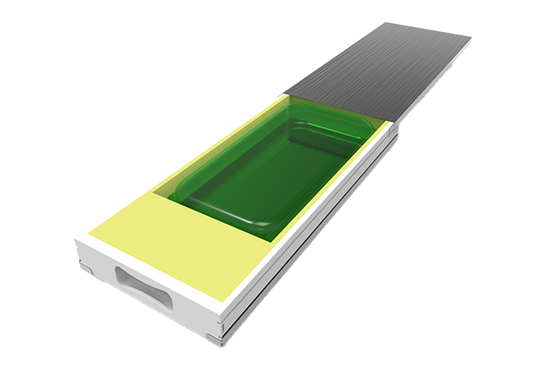
Protecting single-use bags | RoSS® Shell
The safest transport solution for all available single-use bioprocess containers. Protect your single-use bag and reduce product loss. RoSS®: Robust Storage & Shipping
Bag independent
Reduced product loss
Best freezing result
Homogenizing, filling, draining
-
Homogenizing and cooling system | RoSS.PADL
RoSS.PADL is a scalable massaging and mixing system for achieving a uniform mixture in single-use bags. With integrated cooling and heating, it maintains optimal temperatures consistently. This makes it well suited for applications such as cell &a...
Bag independent
Consistent homogeneity
Temperature control
-
Aseptic filling system | RoSS.FILL Bag
RoSS.FILL Bag is a flexible automated aseptic filling machine for the aliquotation and dispensing of bulk drug substance (BDS) into single-use bags. The system for aseptic filling and sterile filtration is highly precise, making RoSS.FILL Bag an e...
Up to 400L+/Batch
Highest accuracy
Fast throughput
-
Draining system for Single-Use Bags | RoSS.DRAI
This standalone single-use bag draining system improves emptying single-use bags on site. Connected peristaltic pumps enable controlled fluid flow and the lowest possible retention volume. Multiple racks can be connected, allowing several single-u...
Up to 60 Bags
Minimum hold-up volume
Aseptically closed system
Freeze, store, thaw
-
Plate freezer biopharma | RoSS.pFTU Large-Scale
Single Use Support's large scale system is a plate-based freeze-thaw unit for any scale and batch size. The system is compatible with single-use bags of all sizes and manufacturers and enables a controlled process down to -80°C of up to 400L per b...
Down to -80°C
Controlled freeze-thaw
Up to 400L+/Batch
-
Blast freezer for pharmaceuticals | RoSS.BLST
RoSS.BLST is a GMP-compatible system for blast freezing & thawing of drug substances. It is suitable for any primary packaging from all manufacturers and allows freezing and thawing within a temperature range of -80°C to +40°C. The modular and...
Capacity for up to 300L
Modular Interior
Down to -80°C
-
Ultra-low temperature storage freezer | RoSS.ULTF
RoSS.ULTF is an upright ultra-low temperature storage freezer for frozen drug substances in different sizes. The ultra cold storage freezer keeps the desired set point temperature down to -80°C. It is compatible with RoSS® Shells to protect your ...
Down to -80°C
Capacity for up to 300L
Modular Interior