Whitepaper: Connecting the Dots - IRIS Single-Use Assemblies

Whitepaper: Connecting the Dots - IRIS Single-Use Assemblies
Filesize: 0.7 MB – Mime-Type: application/pdf
Get full access to this document
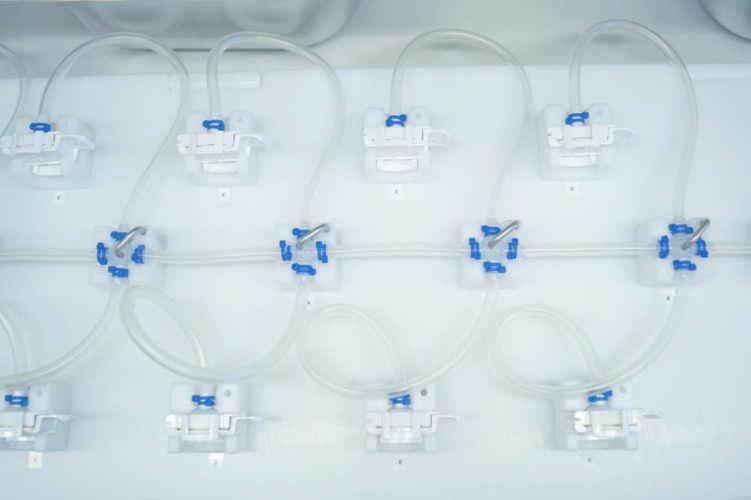
Connecting the Dots: Creation of Single-Use Assemblies for Pharmaceutical Fluid Management
Throughout the entire manufacturing process, pharmaceutical liquids are transferred and have touchpoints within upstream bioprocessing, downstream bioprocessing and fill/finish. Single Use Support provides single-use assemblies of highest quality, reliability and speed of delivery. And with it capabilities in
- Dual Sourcing | Strengthening the Supply Chain
- Customization | Developing off-the-shelf assemblies
- Qualification | Providing GMP-compliant solutions
