Does RoSS® resist vaporized hydrogen peroxide?
Table of contents
ShowInfiltrating product solutions from life science companies or any other supplier into Biopharma environment is justifiably not so simple.
It is the primary goal to avoid contamination and insterility in clean rooms. One of these approaches to infiltrate products is vaporized hydrogen peroxide. RoSS®, the robust storage and shipping container for all 2D single use bags available, has undergone tests to find out whether the treatment of H2O2 has any effect on the surface of all its components.
What is vaporized hydrogen peroxide for?
Decontamination by means of vaporized hydrogen peroxide is commonly used within the pharmaceutical industry. Biopharmaceutical manufacturing, (pre-) clinical trial testing, cell and tissue banks have restricted access through isolators and other barrier systems (cRABS) and transfer hatches (TH).
- The goal is to keep the bioburden as reduced in microorganisms as possible when handling with bio-decontamination agents.
- H2O2 – or vaporized hydrogen peroxide – disinfects such agents
- While being non-toxic, it destroys biological contaminants.
Test results about resistance of RoSS®
The resistance of RoSS and Bottle RoSS with its different components against 3% hydrogen peroxide was evaluated by hollu. The components tested include the packaging foil which comes with RoSS, stainless-steel lids, the PE panels for RoSS and Bottle RoSS and all different kinds of inlay foams - pink, green and yellow.
A Protec Tube+ nebulizer, also used for room disinfection by nebulizing hydrogen peroxide, was used. The above-mentioned parts were treated according to the
manufacturer’s instructions. All samples were nebulized 10 times for 30 minutes with a 3% active aqueous solution of hydrogen peroxide followed by an 2 hours lasting incubation step. In addition, the resistance of the supplied non-packaged foams was determined in a 3-day lasting insertion test.
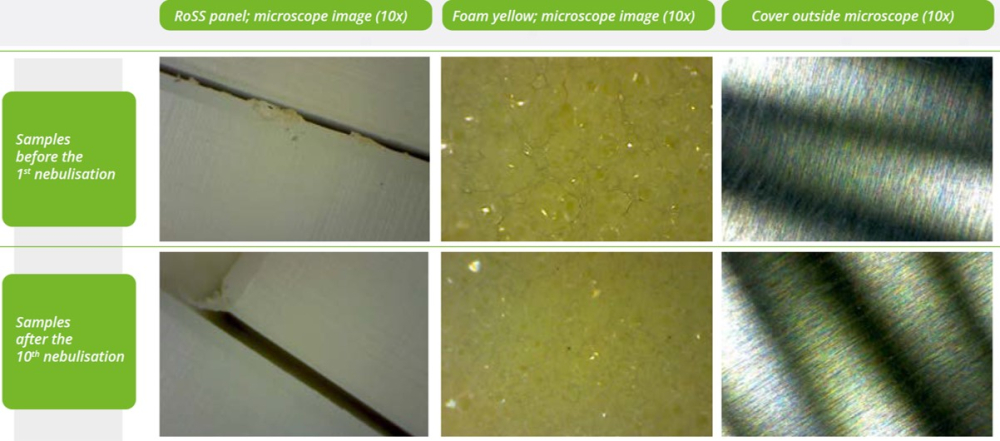
- All tested samples show high stability under the selected test conditions and neither a visual change nor a change in contact angle could be determined.
- As a result, long-term damage caused by decontamination using nebulization and 3% hydrogen peroxide can be excluded with a high degree of probability.
Concluding, RoSS absolutely withstands H2O2 peroxide for decontamination purposes. Even in worst case scenarios. The vaporization of the RoSS shells did not have any effect on the performance of RoSS which was also proven during following freeze-thaw runs at sub-zero temperatures.










