How Hybrid Freeze-Thaw Platforms Defy Cryoconcentration
Table of contents
ShowHybrid solution for drug freezing and thawing involve the use of both primary packagings, single-use bioprocess containers and bottles, the latter also known as rigid containers or PE containers. Biopharmaceutical manufacturers and CMOs typically require separate cold chain management solutions when the drug substance is filled into both single-use primary packagings during bioprocessing.

Advanced freeze/thaw logistics universally applicable
Along with the different shapes of bags and bottles come different freezing characteristics. While 2D single-use bags have a flat design and a low water column, they can freeze faster than bottles. This results in different types of methods for lowering the temperature of drug substance in each primary packaging. While plate freezers provide advanced freezing results, bottles are frozen by blast freezing. Both methods are integrated in hybrid freeze-thaw platforms.
Various studies, including surrogate tests, have been conducted, to evaluate the freezing results of single-use bags and bottles to prevent the effect of cryoconcentration. And since higher freezing rates are also preferred to avoid protein chemical and physical instabilities[1], fast and homogeneously frozen liquids limit cryoconcentration to a high degree, thus enabling highest product quality post-thaw.
Controlled plate-based freezing & thawing of single-use bags
Placing single-use bags in conventional static and blast ultra-cold freezer might be the simplest and most intuitive method for freezing active pharmaceutical ingredients (API). However, with conventional upright or chest freezers it takes several hours to days for all the liquid to reach -80°C. The ice front grows from the outside toward the center of the cryobag. And because water always tries to freeze in its most natural form, it pushes solutes and proteins in its center. This results in the frozen water being separated at the surface and the collected API being trapped in the center. This aggregation – better know as cryoconcentration – can be prevented by controlled and accelerated freezing, such as plate-based freezing.
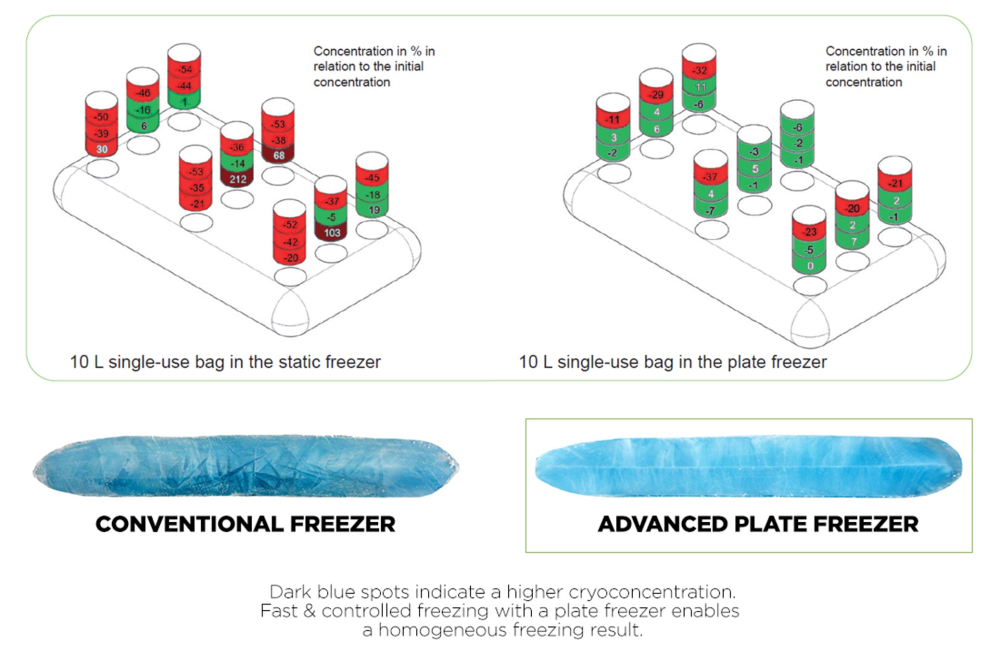
Fig.1: Cryoconcentration in single-use bags frozen in static freezer vs. plate freezer
The comparison of protein concentration between conventional and plate-based freezing is shown in Fig.1. In this study, two 10L single-use bags from the same manufacturer were filled with a protein surrogate solution. The mixture of salt and sugar was specified by a customer of Single Use Support to best mimic the freezing behavior of its product, a monoclonal antibody (mAb) formulation. To visualize the effect of cryoconcentration when drilling and sawing the frozen single-use bag, the color naphtol blue was added, which has no effect on the freezing behavior of the surrogate.
The single-use bag frozen in a static freezer showed a concentration rate of up to 212% in the lower center of the bags, where cryoconcentration occurs in most cases. In contrast, the single-use bag frozen in the plate-based freezer confirmed that cryoconcentration was largely prevented with a maximum concentration rate of 32% inside the bag.
Lower cryoconcentration has been shown to result in higher product quality of API after freezing [2]
Control over freezing and thawing of bottles
Due to their physical shape, bottles are not suitable for freezing on plates. However, there are ways to improve freezing and thawing processes for bottles as well. These are usually frozen in freezers by the air flow from integrated fans.
The combination of the hybrid RoSS.pFTU Large Scale and the use of CCUs (cryo control units) for bottles has enabled homogeneity after freezing surrogates in rigid containers to -80°C, as shown in Fig.2. In this study, two 4L bottles from the same manufacturer were filled with a protein surrogate solution. Again, the color naphtol blue was added to visualize the effect of cryoconcentration.
Homogeneity vs. volcano effect
During the freezing process of liquids in bottles, a “volcano cryo-concentration effect” is often observed (Fig. 2). The accumulation of proteins in the ice formation due to slow freezing results in an upward pressure bulge and a groove in the center of the bottle.
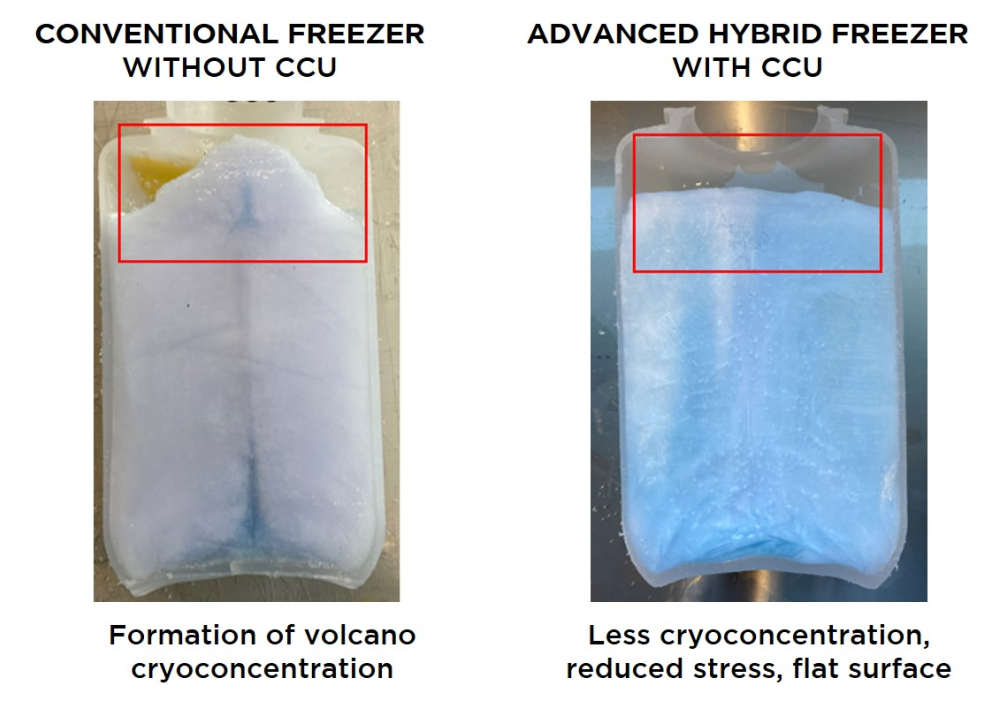
Fig 2: Cryoconcentration in bottles frozen in static freezer without CCU (cryo control unit) vs. frozen in RoSS.pFTU Large Scale Hybrid with CCU
As the name implies, cryo control units (CCU) are devices that are attached to single-use bags and bottles when they are frozen in static or blast freezers to control homogeneity and pressure at the last point of freeze in cryogenic applications in bottles. These vendor-agnostic CCU can be supplied for any type and size of bottle to ensure homogeneous freezing in the hybrid freeze/thaw platform RoSS.pFTU large scale (see Fig.3).

Fig. 3: Cryo control units (CCU) attached to bottles
Bottle thawing process
Plate-based platforms support cooling and heating of pharmaceuticals. So the same method used for freezing can also be applied for controlled thawing of single-use bags.
But what about thawing bottles? They are exposed to a warm air flow to heat liquids. In addition, the RoSS.pFTU Large Scale Hybrid has an integrated shaking function that supports the thawing process of drug substances in bottles. The linear motion which can be adjusted according to the product characteristics, avoids shaking-induced frothing.
Supporting the start of a new era in bioprocess technology
Single-use bags promise to be the primary packaging which ensures the most efficient process and the highest quality in liquid and cold chain management.
Bottles are still widely used due to their low cost of ownership and ease of process integration into manual processes. However, manufacturers and CMOs increasingly face challenges in contrast to bags: scalability and storage capacities due to the bulky shape, more difficult integration into automated workflows, and maintaining product quality during freezing process by avoiding cryoconcentration.
There is a trend towards single-use bags. However, bottles will not be replaced anytime soon. Hybrid solutions support manufacturers to embrace the handling of both, single-use bags and bottles. They offer them additional process flexibility and an efficient footprint by providing an all-in-one platform for state-of-the-art freezing and thawing of drug substance.
References
[1] Minatovicz B. et al.: Freeze-concentration of solutes during bulk freezing and its impact on protein stability. Journal of Drug Delivery Science and Technology 58 (2020) 101703, p.13
[2] Hauptmann A. et al.: Distribution of Protein Content and Number of Aggregates in Monoclonal Antibody Formulation After Large-Scale Freezing. AAPS PharmSciTech (2019) 20:72









