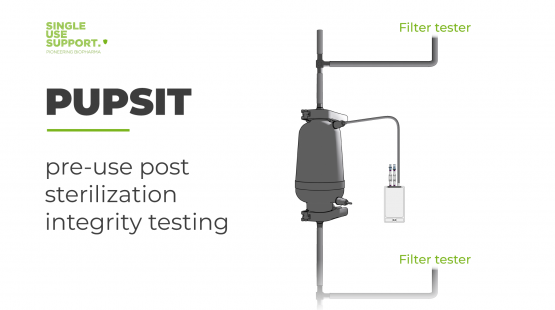
Single Use Support merges product innovation with fast deliveries and customization, offering a wide range of sterile single-use consumables to meet the stringent requirements of the biopharmaceutical industry. Our optimized solutions ensure the integrity, safety, and process efficiency of your biopharma operations. Explore Single Use Support's comprehensive selection of sterile consumables and discover how they can improve your biopharmaceutical workflow.
Our range of sterile single-use consumables include 2D and 3D bioprocess containers, single-use tubing and manifold assemblies, crimping solutions for single-use tubing and aseptic samplers for bioreactors.