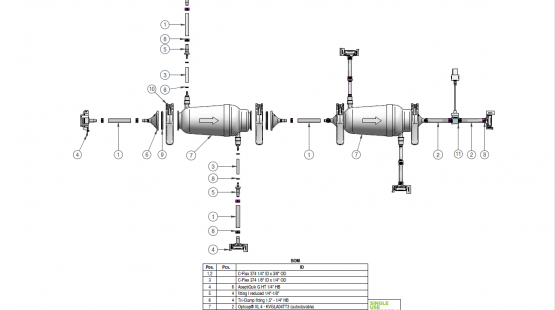
RoSS.PPST is an advanced PUPSIT system that tests the integrity of up to two filters used in biopharmaceutical manufacturing. In accordance with EU GMP Annex 1, it verifies filter integrity after sterilization and prior to aliquotation. With intuitive operator guidance and real-time monitoring of pressure and flow, RoSS.PPST minimizes the risk of errors and contamination.
This automated solution for filtration delivers consistent and controlled results, ensuring safety and quality in your sterile filtration process.