ADC technology simply explained
Table of contents
ShowADC technology is a promising and exciting new approach in cancer treatment, selectively targeting a specific cell surface receptor expressed on cancer cells. With oncology being a main field of application, a number of preclinical tests for treatment of malignancies and solid tumors are currently being conducted.
Their ability to selectively destroy tumor cells makes antibody-drug conjugates highly potent. However, understanding the pharmacokinetics of ADCs is important for optimizing their therapeutic potential.
ADC technology – a definition
By definition, ADC technology is a type of targeted cancer therapy that combines the specificity of mAbs with the cytotoxic effects of chemotherapeutic drugs. ADCs are a common type of bioconjugate designed to selectively bind to cancer cells. This allows them to deliver the small molecule drug – more precisely cytotoxic (anti-cancer) drugs – directly to the tumor cells while minimizing damage to healthy cells.
How do ADC therapeutics work?
ADCs work by connecting a monoclonal antibody with a cytotoxic drug via linker. After having been administered intravenously, they recognize and bind to antigens as target structures on the surface of cancer cells. The toxin is internalized in the cancer cell, where it is then released.
Compared to other types of therapy, ADCs come with less side effects and a more favorable therapeutic index. And because they are also able to act on dormant cells, there is an increasing number of FDA-approved antibody-drug conjugates available, bringing new hope to severely ill patients.
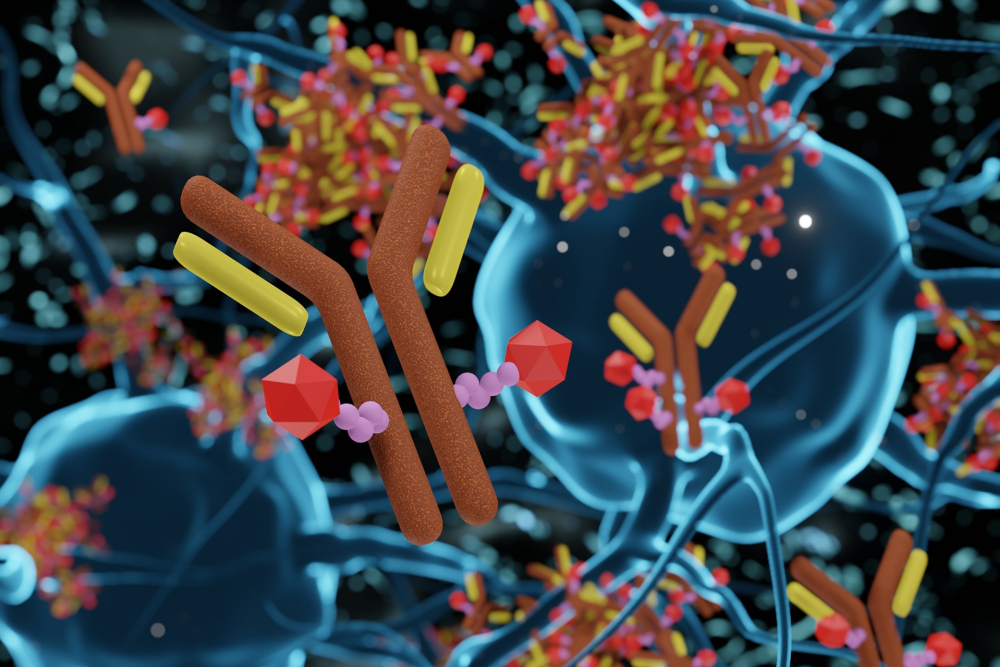
ADC technology – designing Antibody-Drug Conjugates
ADC technology that aims at improving antitumor activity while minimizing side effects for patients and increasing the therapeutic window. At the same time, ADC manufacturing and ADC development leads to the innovation of new classes of linkers that specifically deliver the maximum cell-killing dose to target cells.
Antibody-drug conjugates are complex molecules that are composed of three main components:
- Monoclonal antibody
- Cytotoxic drug
- Linker
Given the right combination, these three components work together to create a highly site-specific and potent therapeutic agent. Having recognized the potential of such agents, antibody-drug conjugate companies produce approved ADCs that can selectively kill cancer cells while minimizing damage to healthy ones. Such companies can be set up as CDMOs, helping in streamlining processes and production in the pharma industry.
Antibody
Antibody-drug conjugates typically use monoclonal antibodies with a high specificity and affinity for a particular antigen.
MAbs are important for the mechanism of action; they are designed to be highly selective, so they can specifically bind to cancer cells while at the same time sparing healthy cells. Any ADC’s antibody component is generally derived from hybridoma cell lines or by using recombinant DNA technology.
Cytotoxic payload
The term cytotoxic payload refers to a type of payload that is designed to induce cell death or destruction upon internalization. The payload range or drug-to-antibody ratio, DAR in short, can vary. It can be a chemical compound, biological molecule, or other agent that targets specific cells or tissues.
The design of the cytotoxic payload is what makes the ADC a powerful therapeutic tool, and there are several commonly used toxic payloads.
- DNA binding cytotoxic agents such as topoisomerase inhibitors
- Tubulin inhibitors such as Maytansinoids
Linkers
Linkers are the component connecting the antibody and the cytotoxic drug. They play a crucial role in determining an ADC’s efficacy and safety. A linker must be stable enough for the drug to remain attached to the antibody in circulation, while it should also be cleavable in the cancer cell to release the cytotoxic drug.
The linker is critical as it must ensure that the drug is released at the optimal rate and in the right location in order to maximize its therapeutic effect while reducing the risk of side effects.
Therapeutic application of ADCs
ADCs have shown promising results in the treatment of lymphoma, leukemia, breast cancer and other solid tumors. They are able to target a variety of antigens expressed on the surface of cancer cells such as CD20 in lymphoma or HER2 in breast cancer.
Examples of antibody-drug conjugates already available on the market include are:
- Adcetris (brentuximab vedotin) for the treatment of Hodgkin lymphoma and systemic anaplastic large cell lymphoma, consisting of an anti-CD30 MAB conjugated to the cytotoxic drug monomethyl auristatin E (MMAE)
- Kadcyla (ado-trastuzumab emtansine) and Enhertu (fam-trastuzumab deruxtecan-nxki) for the treatment of HER2-positive breast cancer, both consisting of the anti-HER2 monoclonal antibody trastuzumab conjugated to the cytotoxic drug DM1 (Kadcyla), respectively DXd (Enhertu)
In addition to these approved ADCs, there are many others currently undergoing clinical trials and in clinical development.
Challenges and chances of ADC technology
Compared to traditional chemotherapy, conjugation technology brings a number of advantages, including improved efficacy and reduced toxicity. However, there are also some challenges that need to be addressed, not only in terms of efficacy and safety, but also regarding their handling.
Safe handling plays a particularly important role when it comes to the general handling, transport and storage of ADCs, as they include toxic substances. Automated systems that facilitate closed processing and put an end to the necessity of cleaning are a feasible and safe solution provided by single-use technologies.
In order to ensure the safety of any operators involved in the manufacturing process, a safe fluid management of ADCs is particularly crucial. To ensure safe production and logistics processes, careful and protected handling should be ensured throughout.
The same is true for controlled freezing of ADCs, which is where Single Use Support’s robust shell system for frozen storage and shipping and its plate-based freezing and thawing platforms comes into play. The protective shell comprises an outer sleeve of robust stainless-steel and an adaptive inner layer of 3D foam. The result is a closed system around single-use systems that can contribute to the safe transport and storage of high-value and potentially harmful substances.