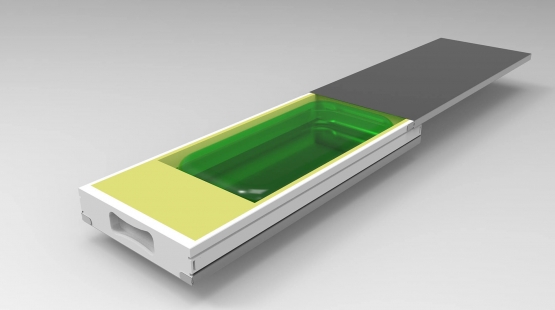
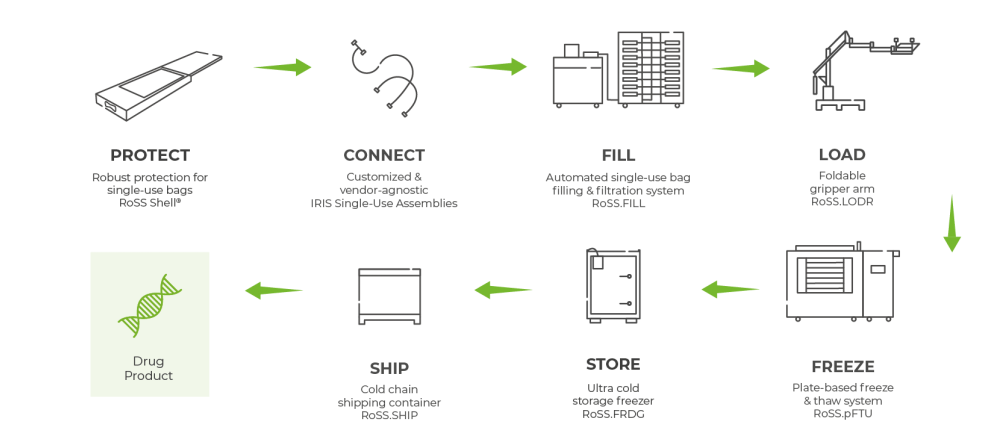
RoSS Technologies provide process flexibility for large volumes at highest quality.
- Closed, robust and tamper-evident protection of your single-use bag for highly valuable drug substances
- Vendor-agnostic and hence compatible to other systems during senstive freezing applications
- Process flexibility for safe handling regardless of type and size of bag manufacturer without being trapped in siloed biopharma process solutions