Advancements in Small Volumes Drug Freezing Techniques
Table of contents
ShowAt the beginning of drug discovery you always deal with small volumes. Handling the cooling of drug substances in small quantities may seem straightforward, yet the intricacies involved are often more complex than anticipated.
Traditionally, the use of a blast freezer or a liquid nitrogen tank has been a go-to solution. However, these methods are primarily designed for cryopreservation temperature maintenance and may compromise the viability of the product when employed for cooling down liquids from ambient to cryogenic temperatures. This is due to their inability to effectively balance the unique characteristics of the products during the freezing process.
The significance of freezing small volumes extends beyond laboratory settings to include commercialized manufacturing processes for small batches. This is particularly relevant for applications like cell banking, gene therapies utilizing viral vectors, lipid nanoparticles, and fill & finish procedures. These processes lay the foundation for subsequent scaling, be it up or out, depending on market demands.
Recognizing the nuanced requirements of different products and implementing tailored freezing methods is crucial for ensuring the integrity and effectiveness of drug substances, whether in the controlled environment of a lab or the dynamic realm of commercial production.
Freezing methods for small volumes
As already mentioned, there are three prevalent methods that are commonly employed for freezing small volumes of cells, biologics, and other active pharmaceuticals ingredients:
- Using a Blast freezer
- Using a Plate-based freezer
- Freezing in a LN2 tank
In the context of blast freezers, achieving ultra-cold storage temperatures is a time-intensive process. The inability to expedite the freezing rate stems from the reliance on cold air. Since the air lacks direct contact with the cooling medium, its impact on the freezing process is limited. Consequently, this method is deemed uncontrolled, as operators cannot influence critical factors such as the freezing rate during phase transition and ice growth based on the product's characteristics.
On the other hand, liquid nitrogen emerges as a common choice for freezing small volumes of cells and other liquids in laboratory settings. Submerging liquids into a liquid nitrogen tank facilitates exceptionally rapid freezing for cryogenic storage below -150°C. Despite the time-saving advantages, this method carries a drawback concerning product quality for numerous drug substances. For instance, optimal cell viability post-thaw is achieved when cells are frozen at a rate of -1°C/min (1K/min). The use of LN2 tanks for instant liquid freezing fails to align with the recommended freezing rate, potentially compromising the quality and effectiveness of the product.
Careful consideration of the trade-offs between speed and quality is essential when selecting a freezing method. Each approach comes with its unique set of advantages and limitations, requiring a tailored choice based on the specific characteristics and requirements of the substances being frozen.
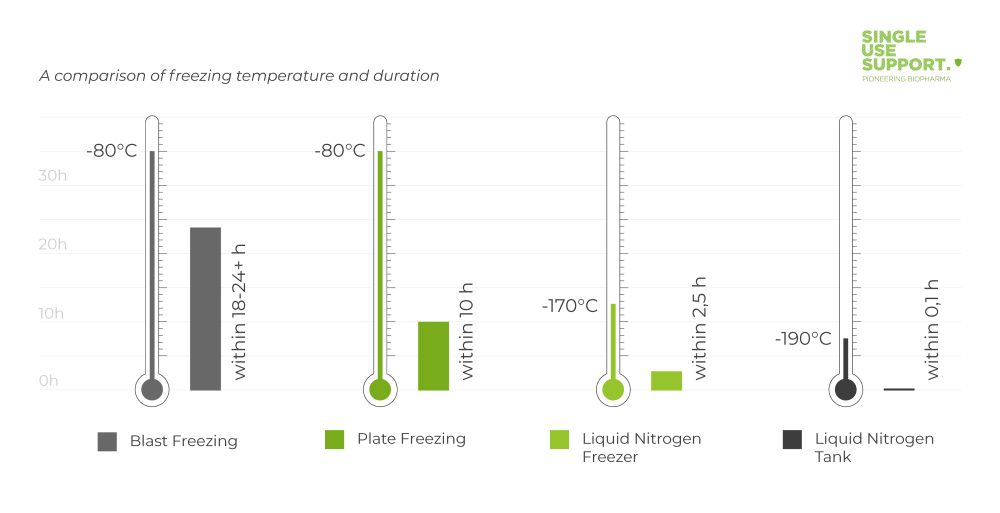
Comparison of freezing techniques
Blast Freezing | Plate Freezing | Liquid Nitrogen Freezer | Liquid Nitrogen Tank |
|---|---|---|---|
(-80°C) | (-80°C) | (-170°C) | (-190°C) |
Cryoconcentration | Homogeneous Freezing in Closed Systems | Controlled freezing rates in bags are adjustable, from 0.5 to 20 K/min | Uncontrolled exposure with too fast freezing |
Limited Scalability | Scalability from 1mL to 1000L+ | Highest product viability | Risk to product viability |
Long process duration | Controlled fast freezing & thawing | Automated process for different scales | Manual, not standardized process |
Exploration of freezing in small volume applications
Initiating the process of professional freezing and thawing for drugs cannot begin too early. Whether in the early stages of drug discovery or during the commercial production of small volumes, controlled freezing becomes an essential element in safeguarding the quality of drug products throughout the manufacturing journey.
Several scenarios highlight the relevance of controlled freezing of small volumes:
- Working cell banks (WCB): Cell banks are required for many different areas of applications. Mammalian CHO cells are required for different cell-based therapies in biomanufacturing. This can be autologous cell therapies, such as CAR-T, or allogeneic cell therapies, emphasizing the need for meticulous control in the freezing process to ensure optimal cell viability.
- Cryopreserved HEK293 Cells for Viral Vectors: HEK293 cells are used as host cells for viral vectors – applied in gene therapies. The controlled freezing of these cells is vital to maintaining their integrity and functionality, ensuring the success of gene therapy applications.
- Non-viral vectors in vaccine production: Vaccine production involves the use of non-viral vectors such as Lipid Nanoparticles and plasmid DNA. Controlled freezing of these components is essential to preserve their efficacy, contributing to the overall success of vaccine manufacturing.
- Cell banking in seed train intensification: Employing controlled freezing in seed train intensification is a strategic approach to enhance bioprocess productivity and efficiency. The optimization of cell banking processes, especially focusing on achieving high cell density, is a decisive aspect for success in advancing upstream bioprocessing.


APP NOTE
"Bestcellers": Controlled Filling & Freezing of Cells
Read more about controlled filling & freezing of cells in our App note: "Bestcellers": Controlled Filling & Freezing of Cells
Transitioning to Precision with controlled rate freezing
In pursuit of successful freezing across diverse applications, the key lies in attaining precise control. While blast freezers and liquid nitrogen tanks lack the flexibility to adjust freezing conditions according to the specific requirements of a product, the adoption of controlled-rate freezing and thawing methods emerges as a transformative approach in optimizing biopharmaceutical productions.
One crucial concern in cold chain management, cryoconcentration, can be effectively mitigated through controlled-rate freezing. Cryoconcentration, characterized by the degradation of product quality due to protein aggregation, often results from the slow growth of the ice front in blast and static freezers. This unwanted effect can be averted by implementing controlled-rate freezing techniques.
The evolving interest in understanding a product's behavior during freezing has gained momentum, particularly in determining the cooling rate at which product viability is maximized. Controlled rate freezing addresses this need, offering tailored solutions such as plate-based freezing to -80°C or liquid-nitrogen-based controlled freezing platforms reaching temperatures as low as -170°C, catering to the specific demands of advanced therapies.
For mammalian cells, optimal freezing rates typically range from -1°C per minute to -4°C per minute, reflecting their comfort zone during the freezing process. Recognizing and adhering to the preferred freezing rate is key in maintaining the viability and functionality of these cells.
Do you know your preferred freezing rate?
Advantages of plate-based freezing
In addition to providing meticulous control over the freezing process to optimize product viability, plate-based freezing brings forth a host of further advantages, making it a preferred choice in the dynamic landscape of biopharmaceutical production.
- Flexibility amidst market dynamics: Volumes might change, size of single-use bags might change. Plate freezers offer a scalable platform that adapts seamlessly to the fluctuating demands of the market and varying volume requirements. Whether dealing with small milliliters or several liters, the scalability of plate freezers ensures adaptability to evolving market needs.
- Process control: All freezing platforms from Single Use Support come along with GMP-compliant software. According to 21 CFR Part 11 it provides audit trails, reports and a full documentation of freezing history to grant for recipe-driven and standardized cold chain management.
- Product quality: The ability to control the freezing rate and ice front growth within the single-use bag translates to elevated product viability. This not only ensures higher product quality but also contributes to a more efficient production process and heightened patient safety.
- Independence from single-use bag vendors: Plate-based freezing excels in delivering scalable freezing performance, accommodating a broad spectrum of single-use bags and volumes, from as little as 10 mL to sizable 50L single-use bags, irrelevant of bag vendors and brands.
- Closed system: A robust and tamper-evident secondary packaging provides a sterile environment for all bags. This safeguards sterility throughout critical stages such as aliquoting, freezing, storing, and shipping, ensuring product integrity from production to delivery.
- Automation: The minimized manual intervention reduces the need for extensive documentation and validation, and shortens process cycles. This increased level of automation contributes to operational efficiency, making the overall production process more streamlined and responsive.

Embracing the control over the freezing for small volumes
The landscape of requirements for each novel therapy is in constant flux, particularly in the dynamic fields like commercial production for viral vectors and cell-based therapies. The freeze-thaw industry has undergone a transformation, and plate-based freezing emerges as the solution that empowers manufacturers to adapt freezing rates, processing volumes, and endpoint temperatures to align with the unique demands of their products. In the pursuit of new treatments, leveraging optimal outcomes is key to the success of Advanced Therapy Medicinal Products (ATMPs).
While static, blast freezers, and liquid nitrogen tanks persist due to historical usage, there exists a pathway to advance cold chain management towards efficiency and precision. Crucially, the controlled-rate freezing offered by plate-based systems holds the promise of not only efficiency but also a significant enhancement in product quality.
The future of freezing and thawing small volumes unmistakably belongs to controlled-rate freezing. By embracing this innovative approach, we also embrace more efficient processes, improved product quality, and ultimately, the continued success of pioneering therapies. The time to advance the freezing and thawing for small volumes in biomanufacturing is now.









