Dry to Die: How Spray Drying can be Replaced by Freezing in the Production of Bulk Intermediates
Table of contents
ShowValuable bulk biopharmaceutical intermediates, such as bacterial or yeast cells in microbial fermentation or other intermediates produced during the manufacturing process of biopharmaceutical drugs, are often transported between different manufacturing sites. Here, it is of utmost importance to maintain their quality while minimizing any degradation in their desired properties. This requires carefully controlled and reliable cold-chain logistics, which in turn relies upon the equally carefully controlled process steps of freezing and thawing.
Spray drying is a widely used method in the biopharmaceutical industry for drying bacterial or yeast cells. However, industry insiders point out that product losses through the process of spray drying of monoclonal antibodies or vaccines often exceed 30%.
The alternative to the industry-accepted spray drying is to freeze the liquids. Until now, freezing such large volumes has been largely avoided with current freezing technologies. Established equipment, such as static and blast freezers, have the disadvantage of long freezing times, resulting in inefficient process solutions and a high loss of product quality due to the effect of cryoconcentration [1].

Novel end-to-end technologies for the freezing, transport, and thawing of biopharmaceuticals are here to advance the handling of pharmaceutical liquids. Plate-based freezing based on single-use systems comes along with improved product quality, lower contamination risk and reduced costs. This optimized ent-to-end cold chain management facilitates biomanufacturers to significantly reduce product losses compared to spray drying and conventional freezing technologies.
Spray Drying Challenges
With spray drying, the mixture containing the compound of interest is atomized and then very hot air is used to rapidly evaporate the water, resulting in a dehydrated powder that can be stored or transported [2]. Spray drying is commonly used to produce solid, particulate proteins for pharmaceutical applications [3], such as inhalable formulations for drug delivery [4], and to produce vaccines that are more stable than those produced using other methods [5]. Bacterial or yeast cells are also commonly spray-dried as an intermediate step in microbial fermentation.
Spray drying of bacterial or yeast cells is an attractive option as it is a relatively fast and scalable process that can produce a fine powder with consistent particle size and good flowability. Spray-dried bacterial or yeast cells can then be stored, transported, and further processed.
However, spray drying is associated with a high loss of product quality. The high temperatures and pressures that occur during spray drying cause large shear forces [6] can lead to considerable process losses due to denaturation or aggregation of the product [5]. Product loss is estimated to be up to 20-30%. Product losses occur with spray drying mainly due to particle deposition on the inner walls of the drying chamber [6]. The limited solubility makes it difficult to reconstitute and use the spray-dried products which are overall more prone to degradation over time. It also remains challenging to apply spray drying to complex, high-value products such as biopharmaceutical intermediates or antibodies [5]. Furthermore, spray drying generally involves an open system, so it is difficult to maintain the aseptic conditions that are crucial during the production of biopharmaceuticals, and the bioburden of the final product can be considerable [6].
In addition, it requires a large footprint and an extensive GMP-compliant facility to implement spray drying into manufacturing capacities. Overall costs of installation of spray dryer and ongoing manufacturing requirements, consisting of maintenance, documentation, validation and manpower, are very high.
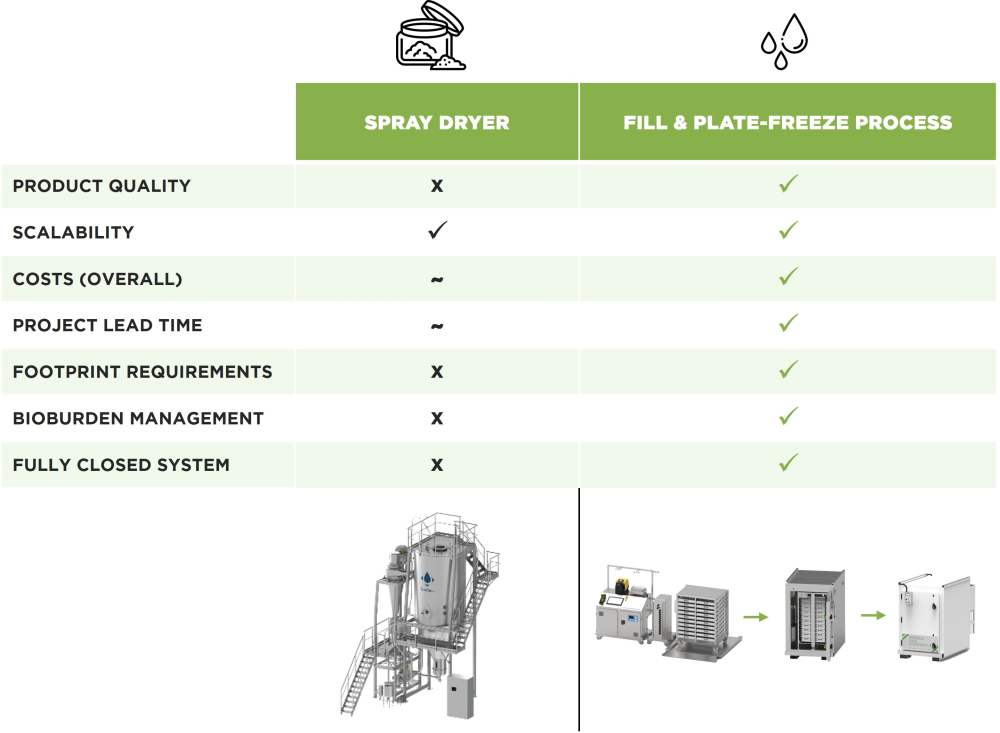
Plate-based freezer technology
When freezing large quantities of protein, i.e., purified protein solutions, the rate of freezing has a major impact on the protein’s stability [7]. However, it is difficult to control the rate of freezing when using the conventional, static freezers employed for this purpose. Biopharmaceutical companies are therefore increasingly turning to alternative, more controllable methods of freezing.
Plate-based freezing and thawing system is one such approach that provides a more controllable method of freezing biopharmaceutical products. Plate-freezers, such as Single Use Support’s RoSS.pFTU system, ensure controlled and uniform freezing, helping to prevent valuable products from degrading during the freezing process. Plate-freezing has proven effective in the controlled freezing of all types of biopharmaceuticals on a laboratory scale but also for larger volumes of up to 1,000L per batch.
Plate-based freezers can be vendor-agnostic when used in conjunction with Single Use Support’s RoSS® secondary packaging range to safely store and transport a frozen product in all available single-use bags. In general, single-use bioprocess containers allow safe liquid transfer in a closed system. Unlike spray drying, the risk of biological contamination is therefore significantly lower.

Controlled freezing refers to controlling the ice front growth speed [1] and controlling the freeze rate [1]. Single Use Support’s plate-based freezing system allows precise control of both these crucial factors, enabling the completely homogenous freezing of samples – an essential requirement when freezing cell suspensions, for example [8]. Controlling the ice front growth speed minimizes the risk of cryoconcentration – the phenomenon that can result in damage to the valuable product being frozen [1]. With some products, a specific freezing rate is required to maximize the preservation of their activity. With plate-freezing, it is possible to control the freezing of 100L of product to freeze at a rate of up to 3°C/minute. Technically, a freezing rate of 5°C/minute is possible when using small bags, but in the case of most biological products such a rate of freezing is too fast. When freezing cells, for example, it is recommended to use a freezing rate of 1°C/minute, so it would take less than two hours to go from +20°C to -80°C.
Advanced plate freezers are universally applicable. They have proven effective when freezing a variety of biopharmaceutical products, including cell-based applications, mRNA, plasmid DNA, and bulk intermediates, such as bacterial cells in microbial fermentation. As another important consideration, plate freezing also facilitates scalability to commercial scales of hundreds of liters per batch.
Advanced single-use technologies facilitate the highly effective filling, freezing, and shipping of large volumes of bulk, embedded within a complete end-to-end infrastructure. Depending on their compliance with US Food and Drug Administration (FDA) 21 CFR part 11, the report and run data are all electronically collected and stored. Uniquely, modular plate-based freeze-thaw platforms allow faster freezing of bulk intermediates or biopharmaceuticals – reducing the time needed even when handling large volumes – and a better-quality product is the result.
Improving manufacturing efficiency
It is increasingly clear that spray drying is not an optimal method for preparing high-value and high-quality biopharmaceutical intermediates for preservation and transport. Product losses associated with spray drying of up to 30% are just unacceptable. Nevertheless, spray drying is widely used in the biopharmaceutical industry. Mainly because there were no suitable alternatives to freezing and thawing.
Single Use Support’s novel end-to-end process solution for filling, freezing, storing, transporting, thawing and draining large volumes eliminates the need for spray drying during the processing of biopharmaceutical intermediates. RoSS.pFTU XL is game changing the industry since it is the first plate-based freezer designed for controlled freezing of large volumes of biopharmaceuticals in a vendor-independent, modular and scalable fluid and cold chain management process.

1. Jenewein, R. and M. Breitrainer, How Controlled Freezing becomes Reality Impact of Ice Front Growth Speed on Scalability of Freezing Protein Solutions (RoSS.pFTU white paper). 2022, Single Use Support.
2. Bakry, A., et al., Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Comprehensive Reviews in Food Science and Food Safety, 2015. 15: p. 143-182.
3. Emami, F., et al., Drying Technologies for the Stability and Bioavailability of Biopharmaceuticals. Pharmaceutics, 2018. 10(3
4. Li, H.Y., X. Song, and P.C. Seville, The use of sodium carboxymethylcellulose in the preparation of spray-dried proteins for pulmonary drug delivery. Eur J Pharm Sci, 2010. 40(1): p. 56-61.
5. Ziaee, A., et al., Spray drying of pharmaceuticals and biopharmaceuticals: Critical parameters and experimental process optimization approaches. Eur J Pharm Sci, 2019. 127: p. 300-318
6. Pinto, J.T., et al., Progress in spray-drying of protein pharmaceuticals: Literature analysis of trends in formulation and process attributes. Drying Technology, 2021. 39(11): p. 1415-1446.
7. Minatovicz, B., et al., Freeze-concentration of solutes during bulk freezing and its impact on protein stability. Journal of Drug Delivery Science and Technology, 2020. 58: p. 101703.
8. Single Use Support, "Bestcellers": Controlled Filling & Freezing of Cells. nd, Single Use Support.









