Steps in Monoclonal Antibody Production
Table of contents
ShowMonoclonal antibodies have kickstarted a new era in diagnostics, therapy, and research and continue to show great potential for use in medicine and science. In this article, we explore the steps that collectively lead to the creation of these biotherapeutics.
From the careful selection of antigens to the strategic utilization of bioreactors, each stage in the production journey significantly contributes to the quality, specificity, and scalability of monoclonal antibodies. With a dedicated focus on cutting-edge advancements, particularly in single-use technologies, we explore how these innovations are shaping the future landscape of monoclonal antibody (mAb) production.
Antigen Selection
The success of the entire process hinges on choosing the right antigen, a molecule capable of inducing an immune response. This carefully chosen antigen will become the target for the antibodies produced, making the selection process a crucial determinant of the ultimate efficacy and specificity of the monoclonal antibodies. The most popular selections are often proteins associated with diseases, and notable examples include HER2 for breast cancer and TNF-alpha for autoimmune diseases.1 2
The choice of antigen influences the functionality and specificity of the resulting monoclonal antibodies. Selecting an antigen relevant to the intended application ensures that the produced antibodies will effectively target the desired molecules or cells.
Several factors come into play when selecting an antigen for monoclonal antibody production:
- Target Biomolecule: The nature of the biomolecule of interest dictates the type of antigen to be selected. Whether it is a protein, peptide, or other molecular structures, the antigen must mimic the characteristics of the target.
- Immunogenicity: The chosen antigen should possess sufficient immunogenicity to trigger an immune response. This ensures the production of antibodies with the desired specificity and affinity.
- Cross-Reactivity: Consideration of potential cross-reactivity is essential to avoid unintended immune responses. The selected antigen should be specific enough to elicit antibodies that do not cross-react with similar molecules.
- Application Requirements: Different applications may necessitate specific antigen characteristics. For example, diagnostic applications may require highly specific antibodies, while therapeutic applications may benefit from antibodies with broader specificity.
Hybridoma Cell Line Development
With the appropriate antigen selected, the next step in monoclonal antibody production is hybridoma cell line development. This process involves the fusion of immune B cells, capable of producing antibodies, with myeloma cells, which have unique properties that allow them to proliferate indefinitely.
Overview of Hybridoma Technology
Hybridoma technology, pioneered by Köhler and Milstein in the 1970s, revolutionized the field of monoclonal antibody production. The process begins by isolating B cells from an immunized organism, typically a mouse or other suitable host. These B cells, responsible for producing antibodies against the chosen antigen, are then fused with myeloma cells, resulting in hybrid cells known as hybridomas.3
Culturing Cells
Once hybridomas are formed, they need to be cultured in a controlled environment. This involves providing the necessary nutrients and conditions for the cells to grow and produce antibodies continuously. The culture medium must support cell viability, antibody production, and the maintenance of hybridoma cell lines.
Screening Processes
The screening of hybridoma cells is a crucial step to identify clones that produce monoclonal antibodies with the desired specificity and affinity. This typically involves testing supernatants from different hybridoma clones to determine their ability to bind to the target antigen. Various screening methods, such as enzyme-linked immunosorbent assay (ELISA) and flow cytometry, help in the identification and selection of high-performing hybridoma clones.
Choosing hybridoma clones that produce high-affinity antibodies is crucial for the success of monoclonal antibody applications. High affinity ensures strong and specific binding between the antibody and its target, enhancing the antibody's effectiveness in various downstream applications, such as diagnostics and therapy.3
Bioreactors in Monoclonal Antibody Production
As we progress in the manufacturing journey, the role of bioreactors becomes increasingly significant. Bioreactors are specialized vessels designed for the cultivation of cells, offering a controlled environment to facilitate large-scale production of monoclonal antibodies. The advantages of utilizing bioreactors include:
- Controlled Growth Conditions
Bioreactors provide precise control over environmental conditions such as temperature, pH, and nutrient levels. This ensures optimal cell growth and antibody production. - Increased Yield and Efficiency
Large-scale production demands higher yields, and bioreactors are designed to maximize cell density and antibody productivity. This efficiency is crucial for meeting the requirements of commercial production. - Aseptic Conditions
Maintaining sterility is paramount in bioprocessing. Bioreactors are equipped with systems to ensure aseptic conditions, preventing contamination and ensuring the purity of the produced monoclonal antibodies. - Continuous Monitoring and Control
Bioreactors allow real-time monitoring of key parameters and the ability to adjust conditions accordingly. Additionally, the systems within bioreactors enable the extraction of samples. This capability facilitates ongoing analysis, ensuring the continuous assessment of product quality throughout the production process. This level of control enhances the reproducibility and consistency of monoclonal antibody production.
Types of Bioreactors
Various types of bioreactors are employed based on specific requirements and characteristics of the cell lines involved:
- Stirred-Tank Bioreactors: These are commonly used for mammalian cell culture and offer efficient mixing of nutrients and gases.
- Perfusion Bioreactors: Perfusion systems provide continuous nutrient supply and waste removal, allowing sustained cell growth and antibody production.
- Single-Use Bioreactors: Increasingly popular for their flexibility and reduced risk of cross-contamination, single-use bioreactors are especially advantageous for smaller-scale productions. However, there are already solutions for larger volumes, too. The largest single-use bioreactors from ABEC or Thermo Fisher Scientific boast volumes ranging from 5,000 to 6,000 L.
Source:4
Monoclonal Antibody Purification
Following successful large-scale production in bioreactors, the next critical step in monoclonal antibody production is purification. Purification involves separating and isolating the desired antibodies from the complex mixture of cellular components, media, and by-products generated during the production process. Several chromatography techniques are utilized to achieve high-purity monoclonal antibodies.
Ensuring High Purity and Yield during Purification
Achieving the desired purity and yield in purification is crucial for the efficacy and safety of the final product. Optimization of parameters such as pH, salt concentration, and temperature is essential to enhance the selectivity of chromatography techniques.
Rigorous quality control checks are implemented to maintain the integrity of mAbs, monitoring concentration, verifying contaminant absence, and assessing overall purity. This dedication to purification excellence, along with careful handling to prevent losses, ensures a final product with high concentrations of pure monoclonal antibodies.
Cell Banking & Seed Train Intensification
Cell banking and seed train intensification are interconnected processes that play a crucial role in ensuring the stability, reproducibility, and scalability of monoclonal antibody manufacturing.
Cell banking involves the preservation of master and working cell banks containing the genetically stable cell lines, such as for monoclonal antibody production. These cell banks serve as a reservoir, providing a consistent, reproducible and faster source of cells over time.
Cell banking in the course of seed train intensification is a strategy employed to enhance cell culture productivity, allowing for increased monoclonal antibody yields. By banking the cell culture in aliquoted single-use bags, it enables manufacturers and CDMOs to achieve a flexible solution for managing working cell banks. Each smaller bag represents a distinct aliquot of the original cell bank, allowing researchers to thaw and initiate cultures selectively upon request.
These processes contribute to the scalability, efficiency, and sustainability of monoclonal antibody production, meeting the demands of commercial applications and advancing the field of biopharmaceuticals.
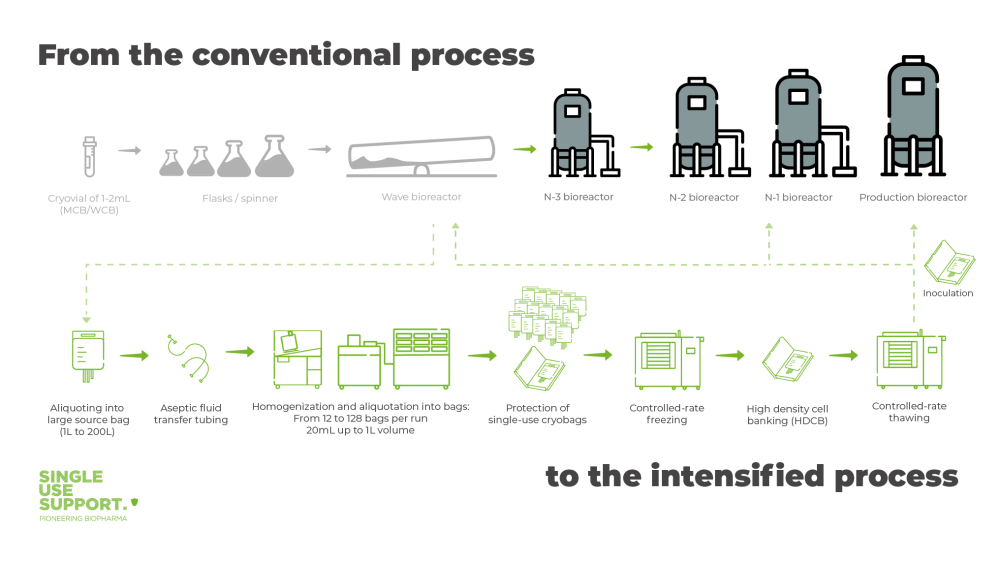
How single-use technology optimizes the steps in monoclonal antibody production
While not a novel trend, the integration of single-use technologies in monoclonal antibody production remains at the forefront of efficiency improvements. The pharmaceutical industry continues to value single-use technologies for their user-friendly, safe nature and profound impact on flexibility, scalability, and sustainability.
Single Use Support’s product lineup offers biopharmaceutical manufacturers comprehensive solutions based on single-use technology. The solutions optimize monoclonal antibody production, implementing automated, closed, and secure production lines encompassing both upstream and downstream processes.
The RoSS.FILL platform facilitates automated fluid management, accommodating varying volumes of biologics under aseptic conditions with high throughput and precision. Fluids are efficiently filled into single-use bioprocess containers and protected by secondary packagings, like the RoSS® Shell. This not only prepares them for the freezing process in the complementary plate-freezing platform but also ensures they are well-prepared for subsequent steps such as storage or shipping.
Recommended articles
- The Role of Tumor-Associated Antigen HER2/neu in Tumor Development and the Different Approaches for Using It in Treatment: Many Choices and Future Directions, https://www.mdpi.com/2072-6694/14/24/6173, Published 14.12.2022
- The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics, https://www.mdpi.com/1422-0067/22/5/2719, Published 08.03.2021
- Hybridoma technology; advancements, clinical significance, and future aspects, https://www.sciencedirect.com/science/article/pii/S1687157X23006236?via%3Dihub, Published 18.10.2021
- Large-Scale Single-Use Bioreactors Can Maximize Long-Term Scale Up, https://www.biopharminternational.com/view/large-scale-single-use-bioreactors-can-maximize-long-term-scale-up, Published 02.04.2022