Pharma 4.0: Pharmaceutical manufacturing on its journey to modernity
Table of contents
ShowPharma 4.0 is the vision of digitalized pharmaceutical manufacturing. The implementation of modern digital strategies into the production of pharmaceutical drugs promises increasing productivity, easier compliance, enhanced connectivity and improved data monitoring to take appropriate action early.
The following insights provide a brief primer on Pharma 4.0 and will convey the key principles of the digital revolution in the pharma sector. In addition, it shows how Single Use Support helps companies in the biopharma industry to overcome obstacles during their adoption to the digital era.
What is Pharma 4.0?
The term “Pharma 4.0” covers the pharmaceutical industry’s striving to implement recent technological advances and to adapt to the new era of digital transformation.
Historically, biopharma companies supplied pharmaceutical substances in a unique regulatory environment with decade-long product life cycles.
By leveraging new technologies in life sciences, the biotech industrial revolution and emerging technologies in the IT sector, the International Society for Pharmaceutical Engineering (ISPE) founded special interest groups (SIG) that provide a roadmap towards a holistic approach to infuse digital maturity into the pharma industry.
ISPE’s Pharma 4.0 vision hinges on digital technologies such as automation, connectivity, big data and data analytics and artificial intelligence. It provides an interconnected framework of resources, information systems, organization and processes, and business culture.
What is Industry 4.0 in pharmaceutical manufacturing?
The fourth industrial revolution envisions a high degree of connectivity, data integrity and process automation through robotics, resulting in workflows with decreasing involvement of human labor. This vision of smart factories encompasses the notion of the industrial internet of things (IOT and IIOT respectively), applying machine learning to production and transportation processes, manufacturing execution systems (MES), control strategies and decision-making.
This operating model integrates holistic data collection among sources such as raw material cost and availability, patient experience, variability in market demands and product characteristics. Pharma 4.0 promises profound improvements in product quality, process control, quality management, and monitoring of supply chains and value chains, hence benefitting companies as well as regulatory bodies such as the FDA and stakeholders in the healthcare system.
Obstacles and security issues
The adoption of new technologies generally comes with associated expenses and novel risks that must be weighed against expected benefits.
First and foremost, the interconnectedness of manufacturing machines with cloud databases highlights the need for adequate data security and protection against digital threats from outside. Additionally, historical databases must be integrated into novel storage technologies and hence, data validation will be a crucial aspect of the adoption of Industry 4.0.
Several processes related to e.g. quality control will be considered as critical and be under scrutiny by regulatory bodies. These key points will increase the strain on IT departments and necessitate adequate resource distributions.
Historically, pharmaceutical companies are expected to be reluctant in adopting to the Industry 4.0 paradigm. Experts from the ISPE expressed that a culture change needs to be established in the pharma sector. In contrast, young and innovative companies strive in this changing environment and offer assistance in adopting and integrating the Industry 4.0 framework. Single Use Support is an early adopter and helps shape Pharma 4.0.

Pharma 4.0 and Single Use Support
The mission of Single Use Support in this “Pharma Revolution” is helping other companies to adapt to Pharma 4.0 by supplying them with innovative products and solutions to overcome their struggles during the digitalization of the pharma sector.
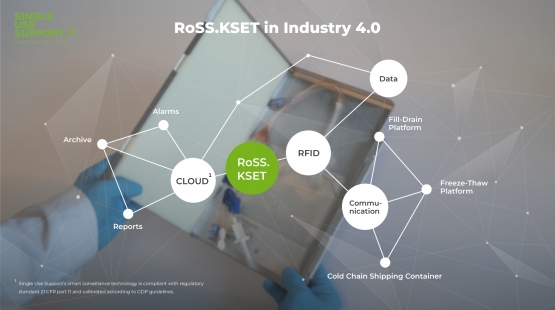
The Single Use Support product portfolio offers a unique fusion of life science and biotech solutions with digital service technologies: RoSS shells protect containers for personalized pharmaceutical products of very high value during the transportation and manufacturing process. In addition, RoSS shells are equipped with RFID tags (radio frequency identification) that enable real-time remote monitoring of crucial parameters (temperature, orientation, impacts) and communication with other products as well as connecting to the Single Use Support Cloud.
This approach facilitates a seamless full lifecycle tracking process and AI-driven data analytics to optimize workflows. Moreover, the Single Use Support Cloud operates as an interface between third parties to exchange critical data, e.g. to orchestrate stakeholders in contract manufacturing organizations, logistics service providers and healthcare centers in the field of Cell and Gene Therapies (CGT).
Pioneering Biopharma
For many years, Single Use Support has attracted the attention of many global pharma companies with its innovations and its drive to shape the world of innovative biopharmaceuticals. For 2022, the goal of the company is to become the forerunner in the industry and to continue PIONEERING BIOPHARMA.