Pharmaceutical cold storage – overview of all freezing ranges
Table of contents
ShowWith the emergence of many innovative approaches in the biopharmaceutical industry in recent years, life sciences companies have needed to put increasing efforts into pharmaceutical cold chain management. Many newly developed, highly sensitive biopharmaceuticals and active pharmaceutical ingredients involved in groundbreaking, life-saving medicine have individual requirements that must take into consideration their safe handling, including storage and transportation.
All relevant stakeholders – from pharma manufacturers to service providers to healthcare providers – demand care in cold chain management, and must therefore pay attention to strict requirements. Here, you will discover why temperature plays an essential role in the entire supply chain of cutting-edge cold chain products. We also guide you through an overview of the most frequently used temperature ranges in freezing drug substances and the long term storage of modern therapeutics.
Pharmaceutical cold chain logistics are a major challenge in the supply chain
The complexity of pharmaceutical supply chains has increased immensely over recent decades, and with temperature-sensitive products leaving pharmaceutical companies to be shipped worldwide to fill-finish sites or warehouses, distributors depend on reliable solutions for cold chain logistics – from packaging solutions to highly advanced refrigeration devices. Equipment reliability and regulatory compliance are key. In addition, there is a high demand to manage the cold chain of vaccines with the highest efficiency, lowest cost and lowest environmental impact.
Regulators such as the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have reacted to the increased complexity that comes with the supply chain of biologics, for example by implementing Good Distribution Practice (GDP) as a regulatory framework for distributors of medicines, to ensure their viability and safety.

Main temperature ranges in cold chain of vaccines
Apart from the ultra-cold environment predominant in a fridge for lab for example (usually -40°C to -80°C ), there are two other important temperature ranges that are used to frame storage conditions for temperature-sensitive pharmaceuticals and active pharmaceutical ingredients under temperature-control, as outlined below.
Ambient temperature
Controlled room-temperature refers to a temperature range between 20°C and 25°C. This, though, does not at all mean that products stored under these temperatures are indifferent to temperature excursions and that temperature control is not necessary.
Refrigerator temperature
A chilled environment, for example in a refrigerator, is characterized by temperatures between 2°C and 8°C and is appropriate for products that must be kept cool but prevented from being completely frozen.
Cryogenic temperature
Products to be stored at cryogenic temperatures require an environment with low to ultra-low sub-zero temperatures. In the process of cryogenic freezing, for example, temperatures can be lower than -150°C, which requires dedicated cooling and storage solutions that go far beyond the capabilities of a household freezer.
As for the importance of the pharma cold chain in the distribution of many medicines and biosimilars, the numbers speak for themselves: In 2015, more than half of the temperature-sensitive products shipped were stored at room temperature, while 31% were refrigerated and 17% were frozen. Additionally, the use of mRNA vaccines as a means to fight the COVID-19 pandemic has further underlined the need for professional cold chain solutions.1
Cold chain management of pharmaceutical products: Temperature requirements
Considering the required temperatures for the storage and transport of various biological products, selecting the exact temperature to be used is in no way an arbitrary choice, but depends on a product’s characteristics. Although the ideal storage temperature may vary within product groups, certain temperature ranges can be clearly given for each of them.
- Monoclonal antibodies and mRNA: stored at -80°C
- Gene therapies: stored at -80°C
- Cell therapies: stored at -130°C and below
- Gene-modified cell therapies: stored at temperatures lower than -150°C
Monoclonal antibodies and mRNA: stored at -80°C
Particularly with the development of COVID-19 vaccines, public awareness of mRNA has grown immensely, along with knowledge of its need for dedicated storage solutions. As it is vital to prevent RNA from degrading, and because some of its components have been shown to still be active at temperatures down to -70°C, a storage environment with temperatures between -20°C and -80°C is considered ideal.
Antibodies have similar requirements for their storage temperature, although the specifics may vary depending on the type of antibody. Generally, however, monoclonal antibodies are stored under liquid nitrogen with temperatures between -20°C and -80°C. In addition to requiring storage at a specific set of temperatures, monoclonal antibodies – immunoglobulins that are made by cloning white blood cells – require fast freezing techniques to prevent cryoconcentration, denaturation, and the formation of aggregates. This is usually achieved using a technique known as plate freezing.
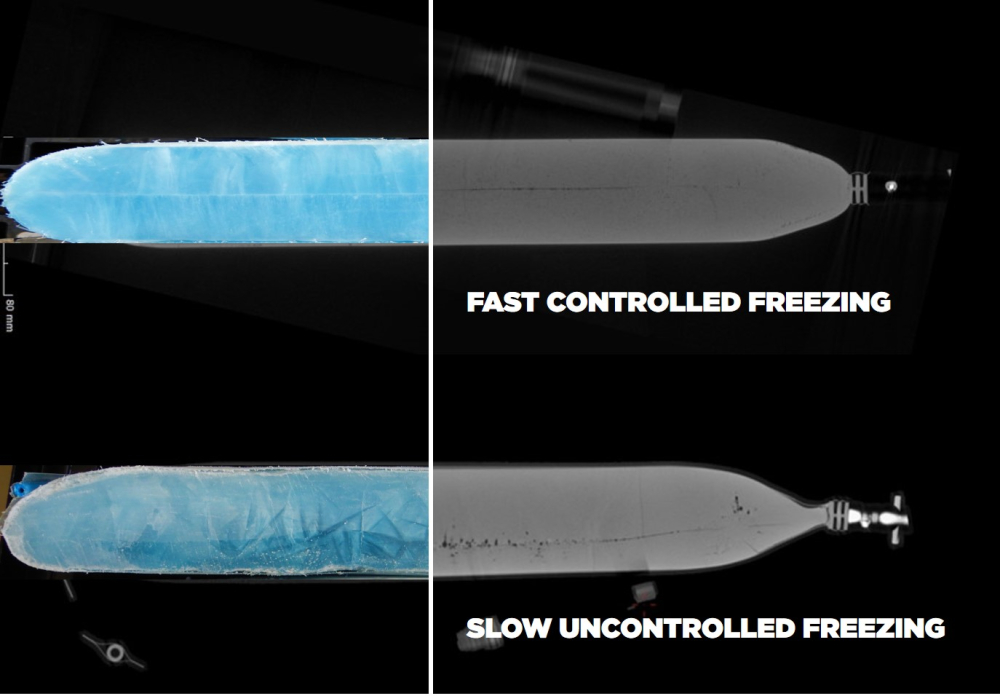
Gene therapies: stored at -80°C
Gene therapies are aimed at either modifying or substituting existing DNA in a cell to fulfill a therapeutic function or to substitute damaged or harmful segments of DNA; this offers new possibilities in the attempt to cure genetic diseases. Vectors are an important tool in the application of gene therapies and are vehicles that are designed to insert DNA into a cell’s nucleus, where the DNA is then read and further processed.
To provide maximum product viability and safety for components used in gene therapy – adeno-associated viruses (AAVs), for instance – storage under ultra-low temperatures is necessary, usually at around -80°C.
Cell therapies: stored at -130°C and below
Cell therapies have enabled huge advances in the fight against a variety of diseases that were previously considered difficult to cure. Cell therapy uses the injection of viable cells – such as stem cells or B cells – harvested either from the patient themself (i.e. autologous cell therapy) or from a donor (i.e. allogeneic cell therapy).
As its name suggests, cell therapy relies on viable cells. These cell therapies are usually derived from a manufacturing process that often involves a long period of time between the harvesting of cells and the actual application of the product. Therefore, appropriate storage is essential and much will depend on how long the product must be stored before it is to be applied. The long-term storage of cell products, for instance, requires cryopreservation at temperatures lower than -130°C if the cell structure is to remain intact.2
Gene-modified cell therapies: stored at temperatures lower than -150°C
Another application for ultra-cold freezing and storage is gene-modified cell therapy. This therapeutic approach involves cells being harvested and genetically modified in vitro prior to their administration to a patient for the treatment of a variety of diseases, including various types of cancer.
There are several types of gene modification used to create cells for therapeutic purposes. For instance, gene modification is indispensable for chimeric antigen receptor T-cell therapy (CAR T-cell therapy) and the production of T-cell receptor therapies (TCR therapies).
Given that gene-modified cell therapies are based on genetic modification performed outside of a patient’s body, the corresponding cells must be kept stable in an ex vivo environment for a certain period. Cryopreservation techniques are frequently used for the long-term storage of these cells at temperatures that are usually lower than -150°C.
Secure pharmaceutical cold chain management with Single Use Support
No matter what the specific needs of biologics are in terms of storage conditions, it is vital to guarantee process safety, efficiency, and product quality along the entire supply chain, and to minimize biopharma supply chain risks as best as possible. Therefore, the biopharma industry relies on smart and dedicated freezing and storage solutions. This is why Single Use Support guides the way with a product portfolio including RoSS.pFTU as a scalable freeze/thaw platform, as well as RoSS.ULTF – an ultra-low temperature freezer that achieves a high level of temperature stability for long-term storage.

In terms of packaging, Single Use Support can yet again be of help, providing secure single-use packaging solutions, such as IRIS single-use bags, as well as RoSS shells, designed to protect your single-use bioprocess containers.
Pharma cold chain logistics – meeting requirements
To meet the requirements of cold chain shipping of biologics, great focus must be put on the appropriate cold chain equipment, e.g. on dedicated transport boxes: Single Use Support has therefore developed RoSS.SHIP – a smart shipping container that can keep temperatures below -60 °C for at least six days. Packing any pharmaceutical materials and drug substances into these highly dense transportation devices help bridge cold chain from manufacturers to pharmaceutical warehouses with ease.
FAQs
What is cold chain management?
Cold chain management in general describes measures that are taken in order to guarantee an intact and uninterrupted cold chain for various perishable goods, such as dairy products, vegetables and other fruit products, but also various pharmaceuticals. Therefore, effective cold chain processes are essential for several sectors in which temperature-sensitive goods are to be dealt with, including public health.
Cold chain operations can vary, depending on the specific needs of a product that is to be transported and stored – from transporting pallets of food in refrigerated trucks, cooling via dry ice or gel packs or cryogenic freezing processes. As for the pharmaceutical industry, the temperature-controlled supply chain management of high quality biopharmaceuticals may include measures like controlled freeze and thaw processes, but also real time temperature monitoring during cold storage and transport in order to avoid disruptions and consequent spoilage.
In order to execute those processes within the cold supply chain in a safe, controlled and cost-effective manner, the biopharmaceutical industry is in need of specialized cold chain technologies, but also trusted partnerships when not all of the biopharmaceutical production steps are located at one site and carried out by the manufacturer. For temperature-controlled vaccine storage and transport, for instance, precise cold chain monitoring is necessary to ensure that manufacturers, shippers, warehousing and storage facilities meet the product’s temperature requirements, maximizing product safety and shelf life, yet minimizing wastage.
What is cold chain management of pharmaceutical products?
Cold chain management in the biopharma industry refers to the range of measures used to ensure the temperature control of temperature-sensitive products. The cold chain of these goods begins at the manufacturer’s site, encompasses transport and storage processes, and ends when the product reaches the patient to whom it is to be administered.
What is the importance of pharmaceutical cold chain?
Cold chain management is essential in the supply chain of various temperature-sensitive products to ensure that they meet high quality and safety standards when they reach their destination. To install cold chain conditions, a temperature-controlled environment is created and maintained, with the aim to avoid processes like deterioration or the undesired growth of microorganisms.
What are cold chain pharmaceutical products?
Cold chain pharmaceutical products are goods that must be stored at a certain temperature to maintain their quality and safety. These goods may be vaccines, components of cells, gene therapies, or biologics.
How do you manage a cold chain?
The cold chain for pharmaceuticals requires uninterrupted temperature-control on the journey from their site of manufacture all the way to where they are administered. As a consequence, dedicated solutions are required for the freezing/thawing, packaging, tracking, and storing of these temperature-sensitive products.
What role does cold chain logistics play in the pharmaceutical industry?
Pharmaceutical cold chain logistics play a crucial role in the production and distribution of several biopharmaceutical products and are thus an important constituent of pharmaceutical supply chains. With regards to the individual requirements of specific products in terms of storage and transport temperature, cold chain management in the biopharma industry requires dedicated cold chain solutions to facilitate storing and transport of highly valuable drug products with a minimized quality loss and safety risks.
- , Published
- Cryopreservation as a Key Element in the Successful Delivery of Cell-Based Therapies—A Review, http://dx.doi.org/10.3389/fmed.2020.592242, Published 2020-11-26









