Scalability of single-use drug substance production
Table of contents
ShowIt is common for early stage clinical products to produce small volumes of drug substance. Traditionally speaking, the use of single-use drug substance bulk freezing containers has been the norm for the 50mL – 1L range. However, while this size offers an acceptable operational fit for dispensing drug substance for smaller- volume pharmaceutical manufacturing processes, it is not ideal for larger-volume dispensing processes.
Most drug substances of interest are valuable, both in terms of their properties and price. It is not feasible to freeze large quantities just to determine the right freezing conditions.
Hence, scalability is important. The idea is to freeze small quantities in the range of 0.5L to 10L and determine optimal freezing conditions that can later be scaled up to larger systems (up to 100L).
The aim was to achieve a freezing process where the last point of freeze always happens at the same time throughout all scales. If successful, the generated freezing curves will look very similar. Temperature ramps have been used to slow down or accelerate the freezing process.
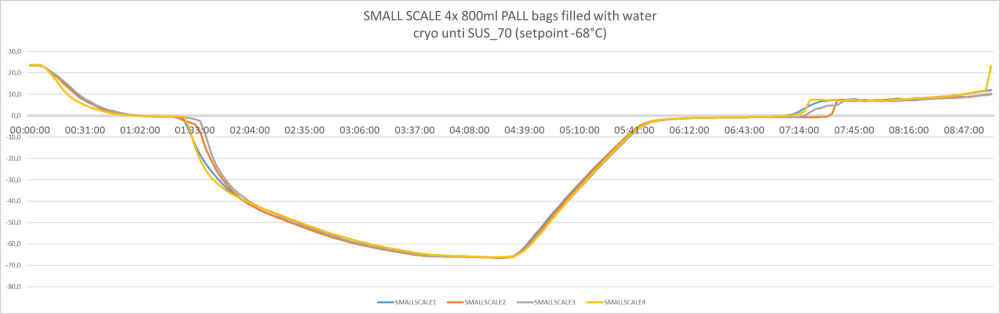
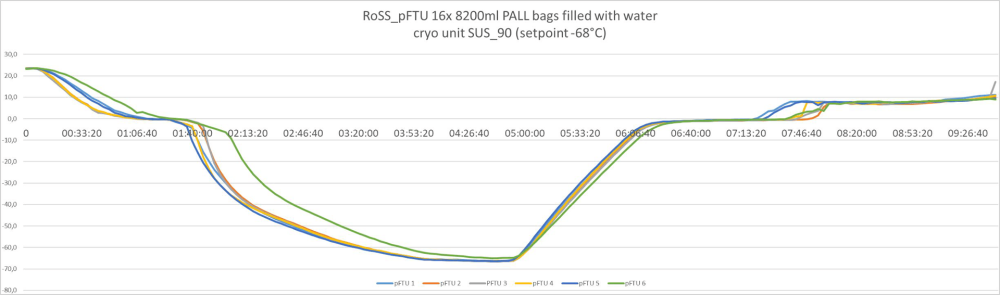
The results of the test, scaling up from 3L to 300L are illustrated in the figure below.

What’s scalability?
- Scalability around freezing means the ability to guarantee constant stress on proteins (biopharmaceutical molecules) in all scales, filling volumes and loading scenarios.
- Single Use Support fulfils this requirement by standardizing the IFGS – ice front growth speed – for all possible scenarios regardless of the filling volume and type of bags but also regardless of the type of system, i.e. whether RoSS.pFTU Lab Scale or Large Scale.

Batch size volume and load independent homogeneous freezing and thawing results:
(example of freeze/thawing curves of a fully scalable process)
3 liters / pre-clinical or Phase 1/2
150 liters / Phase 2/3 or commercialized
300 liters / commercialized


Freezing kinetics must be practically identical no matter if you freeze 500mL or 200L.
Johannes Kirchmair, CEOIt is typical for commercial programs to generate several hundred liters of drug substance per batch.11 In general, the awareness and importance of the gap in bulk drug substance management (between downstream and fill and finish) is steadily increasing. Biopharmaceutical companies are willing to invest more in relevant technologies. This shows that securing the highest possible standard is not only important in terms of efficiency, but also in order to safeguard high-quality products as a responsibility towards patients.12
[Sources from the SUS_ebook_Rev. 1]









