7 Must-Known Facts About Antibody-Drug Conjugates (ADCs)
Table of contents
ShowAntibody drug conjugates (ADCs) are a rapidly growing field of interest in biopharmaceutics. In comparison to conventional chemotherapy treatments, ADCs have the potential to destroy cancerous cells without damaging healthy cells in the process.
Through a monoclonal antibody that is linked to the cytotoxic drug, it becomes possible to target cells more efficiently and deliver the cytotoxic drug where it is needed for lysosomal degradation and kill cancer cells. Because of this mechanism, ADCs are also referred to as “biologic missiles”. Several ADCs are already successfully used in the treatment of myeloid leukemia or refractory metastatic breast cancer. Many others are in clinical trials and waiting for their FDA approval.
Recent advances in ADC development have further enhanced their precision and effectiveness, opening up new possibilities for their use in cancer therapeutics. In this article we will give you an introduction to this important and rapidly growing field. We will inform you about the different components and functions of ADCs and provide you with seven facts you probably didn't know yet about antibody drug conjugates.1 2
Antibody Drug Conjugate Definition
Antibody drug conjugates are highly effective drug substances used in the treatment of various cancers. They consist of a combination of a monoclonal antibody (mAb) and a small molecule drug which have been connected through a chemical linker. This innovative approach in drug development targets cancer cells with high precision.
There are only a few known payloads that are stable and potent enough to be suitable for ADCs, yet. Often payloads are used that are too toxic to be used as a stand alone drug. One example is monomethyl auristatin A MMAE, which inhibits microtubule polymerization in the cancer cell’s cytoplasm. Further, there are cytotoxic drugs which involve amatoxins, anti tubulin (MMAF) and alkylating agents. It is important to regulate pharmacokinetics and selectivity to ensure a successful conjugate without unwanted “bystander killings” of healthy cells.
Read more:
Bioconjugation simply explained
ADC technology simply explained
While the optimization of the antibody and drug conjugate facilitates to target cancer cells more efficiently without causing undesired side effects like damaging normal tissue, these are still common problems in conventional chemotherapy treatments. This is why ADCs have a promising future ahead. Many pharmaceutical companies therefore invest a lot of money and time in pushing progress in the research and clinical development of improved antibodies and efficient manufacturing techniques.3
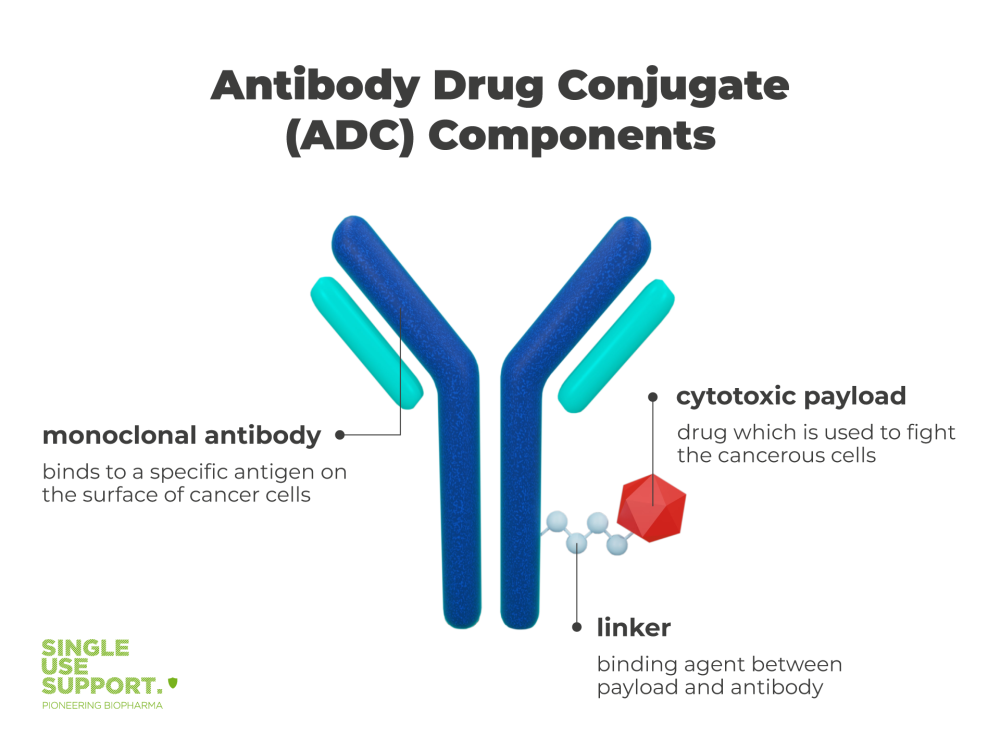
1 Antibody Drug Conjugate Components
Fact 1: There are three main components of antibody drug conjugates.
There is the antibody, the drug or payload and the linker. This is how they work:
The antibody
Antibodies have the ability to target potential threats through high binding specificity to an antigen. They are able to bind to proteins and receptors on the cell surface of cancer cells.
Payload
The drug which is used to initiate the desired response and fight the cancerous cells is also referred to as cytotoxic payload, like calicheamicin or maytansinoids for example. It is released once the antibody has docked onto the tumor cell.
Linker
The non-cleavable linker is the binding agent between payload and antibody. Its stability is an important factor in the ADCs efficacy and has to be able to be maintained even in precarious conditions to make sure the cytotoxic agent can be delivered to the cancerous cells. At the same time it has to be able to release the payload once the antibody has bound to the cancer cell by disulfide reaction. The linker also needs to be able to release the payload once the antibody has bound to the cancer cell. This process often involves enzymatic activity, as certain linkers are designed to be cleaved by specific enzymes present within the cancer cells. 4
2 Mechanism of Action
Fact 2: Antibody-drug Conjugates selectively kill cells.
An antibody drug conjugate is the combination of mAbs’ highly specific targeting and cell killing abilities of cytotoxic agents in anticancer drugs. To fight cancer most efficiently without harming healthy cells in the process, an targeting antibody is coupled with the drug through a chemical link. This causes higher malignancy selectivity while improving drug tolerability.
As soon as the mAb recognizes the potential threat, it targets the cancerous cell. For circulating ADCs to recognize it, the antigen should ideally be a surface or extracellular antigen, rather than an intracellular one. Then the peptide binds to the specific target antigen on the surface. Through this mechanism of action a reaction is triggered. The cancer cell receives the peptide signal and absorbs the antibody linked to the cytotoxic agent. After the internalization of the ADC, the cytotoxic agent initiates cell death.5 6
3 FDA Approved Antibody-Drug Conjugates
Fact 3: There are currently twelve products for cancer treatments that have gained the FDA certificate.
Due to new possibilities through technological advancements, a rapidly growing number of ADCs is currently in preclinical development or in clinical trials and pharmacologic evaluations, waiting for their FDA (food and drug administration) approval. ADCs offer promising treatments for various types of cancer, including lung cancer, gastric cancer and breast cancer, by targeting tumor cells while minimizing damage to healthy cells.
These novel therapies carry certain risks, such as potential side effects, including thrombocytopenia (low platelet count), which can impact blood clotting and overall patient safety. Nevertheless, ADCs represent a significant advancement in targeted cancer therapy, providing new avenues for more effective and safer treatment options.
As ADCs are a relatively new class of drug, more approvals are expected in the near future. Below, you can find the names and producers of approved ADCs.
- Gemtuzumab ozogamicin (Pfizer/Wyeth)
- Brentuximab vedotin (Adcetris®, Seattle Genetics, Millenium/Takeda)
- Trastuzumab emtansine (KADCYLA®, Genentech, Roche)
- Inotuzumab ozogamicin (Pfizer/Wyeth)
- Polatuzumab vedotin (Genentech, Roche)
- Enfortumab vedotin (Astellas/Seagen)
- Trastuzumab deruxtecan (Enhertu®, AstraZeneca/Daiichi Sankyo)
- Sacituzumab govitecan (Trodelvy®, Gilead Sciences)
- Belantamab mafodotin (Blenrep®, GlaxoSmithKline)
- Moxetumomab pasudotox (Lumoxiti®, AstraZeneca)
- Loncastuximab tesirine (Zynlonta®, ADC Therapeutics)
- Tisotumab vedotin-tftv (Tivdak®, Seagen Inc. and Genmab)
- Mirvetuximab soravtansine (Elahere™, ImmunoGen Inc.)
4 Manufacturing of ADCs explained
Fact 4: Antibody drug conjugates are not so easy to make.
Antibody drug conjugate manufacturing is a highly complex and laborious process which includes not only antibody production, but also chemical synthesis and ADC conjugation. To explain the overall concept in a simplified manner, the complicated trial can be broken down to these steps:
1. Antibody production: The antibody with the desired qualities is manufactured and expressed in a host cell line (i.e. hybridoma cells, mammalian cells or microbial cell lines).
2. Chemical synthesis: Linker molecules are developed and modified to achieve a high level of stability which is needed in critical pH conditions and different serum protease inhibitors.
3. ADC conjugation: The site-specific conjugation of the drug and antibody is achieved in a reactor. The ADC is then filtered and purified through chromatography systems to get rid of any impurifications that could change the efficacy of the finished product. In the finishing step the ADC is filled into aseptic bioprocess containers.
The drug to antibody ratio (DAR) is an important point to consider in the manufacturing of ADCs to guarantee their efficacy. During the whole process current good manufacturing practices (cGMP) have to be operated at all times to guarantee a safe and effective product. In vitro testing plays a crucial role in evaluating the ADC’s effectiveness, stability, and potential side effects, allowing for the optimization of the manufacturing process. If any impurities remain or a part of the ADC is contaminated during the process, the safety of the drug for the patient can no longer be guaranteed.

5 Companies in ADC manufacturing
Fact 5: Pfizer, AstraZeneca, Gilead Sciences & Seagan and many more are big pharma manufacturers for antibody drug conjugates.
The production and research on ADCs have quickly become a rapidly growing field of interest. Targeted oncology therapeutics have become one of the most promising sectors in the biopharmaceutical industry due to their advantages over common chemotherapeutic treatments.
There are several companies which play a key role in the research, development and production of ADCs. To name just a few of the most influential companies in ADC production, Pfizer, AstraZeneca, Gilead Sciences and Seagan are among the names that have to be mentioned.
Pfizer Inc. was one of the first providers of ADCs and continues its ambitious development of antibodies for this purpose. Just as AstraZeneca, their main focus remains on oncological drugs. As the second leading patent filer for ADCs on the market, Gilead Sciences continues as one of the most influential companies in ADC development. Further, big advances on the treatment of metastatic urothelial cancers have been made through a collaboration by Seagan and Astella Pharma.
6 ADCs cytotoxicity as a challenge
Fact 6: ADCs cytotoxicity is a big challenge in manufacturing.
The many benefits of ADCs are however associated with a big risk due to the extreme toxicity. ADCs are categorized as highly hazardous pharmaceutical products due to cytotoxicity of their payloads.
As this poses a major health threat on the staff involved in the process, ADCs have to be operated in securely closed systems at all times. ADC production has to follow strict cGMP standards to guarantee safe manufacturing practices for staff and product use for cancer patients.
Further, it relies on aseptic processing and filling techniques. To fulfill all of these requirements, a lot of time and money has to be invested. To take the strain off staff and supply chains, single-use technologies are used by many of the key CDMOs or CMOs.
7 Safe handling of Antibody Drug Conjugates
Fact 7: Single-use technologies improve antibody drug conjugate manufacturing
To make sure drug efficacy is fully functional and the chemotherapeutic drug is free of any impurities, aseptic production has to be a given in the manufacturing of ADCs. This includes aseptic production tools and filling devices and an aseptic environment (clean room). These standards make the manufacturing of ADCs a cost intensive and laborious process. As the process is prone to human errors, the risk of product loss increases the pressure on manufacturers.
Single-use technologies help to improve safety conditions for staff and streamline the process in order to prevent possible bottlenecks. Through fully automated solutions and fluid management with single-use equipment it becomes possible to manufacture and finish products without exposure to the environment. The risk of human error and accidental contact with hazardous substances is reduced to a minimum.
More readings about safe processing in ADC manufacturing:
Safe filling and aliquoting of ADCs
Controlled freezing of ADCs
Process Safety in fluid management of ADCs
Outlook for Antibody Drug Conjugates
With the many advantages ADCs bring to the table in the treatment of solid tumors it does not surprise that progress in the research for new molecules as well as antibodies with higher specificity lies in the interest of many pharma companies.
Especially for diseases like B-cell lymphomas, a high specificity in antitumor activity is extremely important to combat the disease efficiently. Further, CMOs are always on the lookout for new and improved production techniques for cytotoxic agents. As the efficacy of ADCs also relies on the drug to antibody ratio, controlling the homogeneity of ADCs is another focus for many CMOs.
However, there are only a few CMOs who are able to produce ADCs efficiently. To overcome challenges in manufacturing due to the cytotoxicity of the drug and the potentially slow rate of antibody production, leading key players in this field rely on single-use technology.
As most CMOs have specified in one aspect of ADC manufacturing, often many partners are needed in the production. This can lead to bottlenecks in the supply chain and - in consequence - to production loss because time plays an important role in the half life of antibodies. However, as the sector keeps on growing and new companies are entering the market, this problem will be overcome.
FAQs about Antibody Drug Conjugates
What are Antibody Drug Conjugates (ADCs)?
Antibody drug conjugates (ADCs) are targeted cancer therapies that combine a monoclonal antibody with a cytotoxic drug through a chemical linker. The antibody specifically binds to antigens on cancer cells, delivering the cytotoxic drug directly to the target cells, which enhances the drug's effectiveness and reduces damage to healthy cells.
What are examples of antibody drug conjugates?
Some examples of antibody drug conjugates (ADCs) include Brentuximab vedotin (Adcetris®), which is used for treating Hodgkin lymphoma and certain types of non-Hodgkin lymphoma. Trastuzumab emtansine (KADCYLA®) is another ADC used for treating HER2-positive breast cancer. Additionally, Sacituzumab govitecan (Trodelvy®) is used for treating triple-negative breast cancer. These ADCs represent the innovative targeted therapies available for various cancers, providing more precise and effective treatment options.
What is the therapeutic index for ADCs?
In antibody-drug conjugates the therapeutic index refers to the ratio between the maximum tolerated dose of the cytotoxic payload derivative and the minimum effective dose required for the desired therapeutic effect. ADCs have a narrow therapeutic index due to the potent nature of the payload, and therefore require careful dosing and monitoring to ensure safety and efficacy.
Are ADCs used as Anti-HER 2 therapies?
ADCs are used as anti-HER2 therapies. ADCs can target the HER2 protein on cancer cells and deliver a cytotoxic drug to kill them. There are currently several anti-HER2 ADCs approved for the treatment of HER2-positive breast cancer, including T-DM1 and trastuzumab deruxtecan.
Are ADCs an immunotherapy?
ADCs are not considered traditional immunotherapy, as they do not directly stimulate the immune system. However, they are a type of targeted therapy that can be used in conjunction with immunotherapy to enhance treatment efficacy.
What was the first FDA approved ADC?
The first FDA-approved ADC was gemtuzumab ozogamicin, which was approved in 2000 for the treatment of acute myeloid leukemia. However, it was later withdrawn from the market and then reintroduced in 2017 with new dosing recommendations.
How does the immunogenicity of ADCs impact their safety and efficacy?
Immunogenicity refers to the potential of a therapeutic to trigger an immune response in the body. In the context of antibody drug conjugates (ADCs), the immunogenicity of the monoclonal antibody or the linker-drug complex can affect both the safety and efficacy of the treatment. High immunogenicity may lead to the development of anti-drug antibodies (ADAs), which can reduce the effectiveness of the ADC by neutralizing its activity or accelerating its clearance from the body. Moreover, an immune response could result in adverse effects such as allergic reactions or inflammation. Therefore, minimizing immunogenicity is crucial for the success of ADC therapies and requires careful design and selection of ADC components during development.
How are bioconjugates manufactured?
Bioconjugates are typically manufactured through a process called bioconjugation, which involves attaching a payload molecule to a monoclonal antibody (mAb) or other biologic via a linker. This process can be performed using a variety of techniques, including chemical conjugation, enzymatic conjugation, and genetic engineering.
What is the bioconjugation process?
Bioconjugation is the process of covalently linking two or more molecules together, typically a protein such as a monoclonal antibody (mAb) or a small molecule drug, to create a single entity with enhanced therapeutic properties.
Articles about ADCs
- Antibody–Drug Conjugate (ADC) Clinical Pipeline: A Review, http://dx.doi.org/10.1007/978-1-62703-541-5_1, Published 2013-08-01
- Antibody-drug conjugates as novel anti-cancer chemotherapeutics, https://pubmed.ncbi.nlm.nih.gov/26182432/, Published 12.06.2015
- Unlocking the potential of antibody–drug conjugates for cancer therapy, http://dx.doi.org/10.1038/s41571-021-00470-8, Published 2021-02-09
- Drug-Linkers in Antibody–Drug Conjugates: Perspective on Current Industry Practices (Zhang), https://colab.ws/articles/10.1021/acs.oprd.3c00136, Published 29.06.2023
- Antibody drug conjugate: the “biological missile” for targeted cancer therapy, http://dx.doi.org/10.1038/s41392-022-00947-7, Published 2022-03-22
- Antibody‐drug therapeutic conjugates: Potential of antibody‐siRNAs in cancer therapy, http://dx.doi.org/10.1002/jcp.28490, Published 2019-03-25
- Antibody–Drug Conjugates: Future Directions in Clinical and Translational Strategies to Improve the Therapeutic Index, http://dx.doi.org/10.1158/1078-0432.CCR-19-0272, Published 2019-04-12
- Antibody-Drug Conjugates in Cancer Therapy, http://dx.doi.org/10.1146/annurev-med-050311-201823, Published 2012-10-09
- Biopharma PEG, https://www.biochempeg.com/article/74.html, Published 07.05.2024