Cell viability & viability assays: 7 facts to be aware of
Table of contents
ShowCell viability is an important topic in cell banking, as it expresses the amount of healthy cells in a sample and is counted among the most important CQAs (Critical Quality Attributes), determining the quality of biological products. In order to ensure a cell line’s health, various parameters must be considered and in-vivo or in-vitro testing has to be conducted.
Why does cell viability matter?
By providing important information about cell health, cell viability helps researchers determine whether said cells are capable of carrying out essential biological processes. This is crucial in cGMP cell banking, as it helps determine the efficacy of treatments as well as the success of cell-based applications. Apart from informing life science researchers about possible apoptosis, it is a prerequisite for advances in medicine and biotechnology.
What is a cell viability assay?
A cell viability assay is a laboratory technique used to determine the number of viable (living) cells in a given population. It assesses the ability of cells to survive and proliferate under specific conditions or after exposure to various treatments such as drugs, chemicals, or environmental factors. Cell viability assays typically involve staining cells with dyes or fluorescent markers that selectively label living cells, allowing researchers to distinguish them from non-viable cells. This technique plays a crucial role in various fields including biomedical research, pharmaceutical development, and toxicology, providing valuable insights into cell health, function, and response to external stimuli.
Fact 1: Cell viability expresses a ratio
Cell viability “is the quantification of the number of live cells”1 and describes the number of healthy cells in a sample. In other words, It refers to the number of viable cells compared to the total number of cells within a cell population. Common methods used to determine and measure cell viability ratios include flow cytometry as well as various dyes, such as Trypan blue.1
Fact 2: Cell proliferation can outweigh loss of cell viability
Cell viability expresses cell health and survival, while cell proliferation refers to the ability of cells to multiply, and is an important aspect of cell growth and tissue development at the end of a cell cycle.
While BrdU is an important marker used to detect actively proliferating cells in culture or tissues in real-time, proliferation can also be tested by assays such as DNA synthesis assays, multiplexing, Formazan products or cell counting.
Although cell proliferation is able to outweigh loss of cell viability to some extent, it is worth mentioning that the number of times a cell can proliferate is usually limited. Therefore, cell viability is ideally maintained high in the first place.
Fact 3: Cell viability ≠ cell recovery
Cell viability is typically assessed at a specific point in time, whereas cell recovery focuses on the cells’ ability to recover after stress, which correlates to dehydrogenase, or experimental manipulations. If viewed together, those two measurements offer a more comprehensive understanding of the behavior of live cells and the effects of various factors on cell health and function. Assay kits used to measure cell recovery include, among others, MTT assays or Trypan blue.
Fact 4: Cytotoxicity and cryoconcentration can reduce cell viability
While different cell types have varying sensitivities to environmental conditions, they can all be affected by factors like cytotoxicity and cryoconcentration. Nevertheless, when freezing cells, one has to consider various Critical Process Parameters (CPPs), i.e. parameters within a manufacturing process that can influence CQAs (Critical Quality Attributes).
Cytotoxicity
This type of toxicity will eventually lead to cell damage, impaired cellular function, and, finally, apoptosis. Exposure to certain reagents or environmental toxins can induce cytotoxicity, which is why cytotoxicity assays are essential to rule out any toxicity that could lead to lysis.
Cryoconcentration
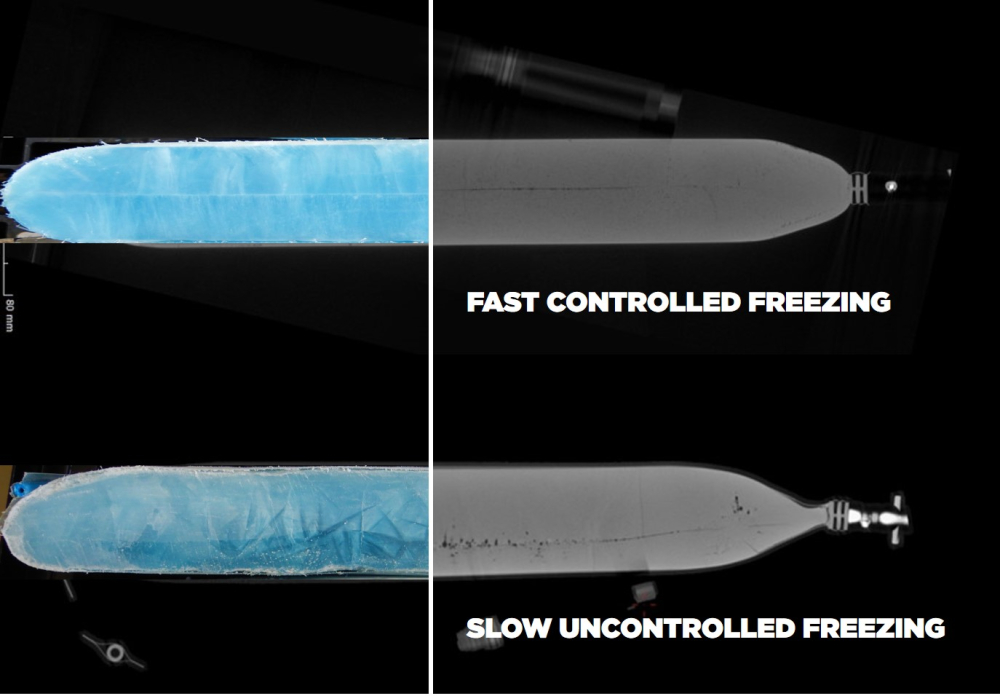
If not monitored, cryoconcentration, also known as freeze concentration, can reduce cell viability. This can be facilitated by a too slow freezing process and either happens through osmotic stress or the formation of ice crystals that will damage cell membranes and cellular structures as they grow. Colorimetric assays are used to measure membrane integrity.
Fact 5: There are different types of cell viability assays
Cell proliferation assays and viability assay kits are used to investigate the metabolic activity of cell cultures after exposure to stimuli or toxicity.
Apart from cytotoxicity and colorimetric assays, there are other cell viability assays, all of which employ specially designed reagents to determine cellular function, such as enzymatic activity (enzyme activity substrates), or metabolic activity. The following are some of the most important assays:
Luminescence-based assays
Including ATP assays (adenosine triphosphate assays) and luciferase reporter assays, they are widely employed to measure cellular metabolic activity and the expression of specific genes or reporter constructs in live cells. Utilizing luminescent signals, these assays are used to detect and quantify the levels of ATP or the activity of luciferase enzyme.
Tetrazolium salts
Tetrazolium salts, such as WST-1, MTS, XTT or MTT assays, are commonly used to assess cell viability, proliferation and metabolic activity. They are colorless compounds that are reduced by metabolically active cells to form colored formazan products, with the color’s intensity being directly proportional to the number of viable cells and their metabolic activity.
Colorimetric assays
This type of assay can be visually examined under light microscopy and makes use of well plates or microplate readers, with the general principle being similar to ELISA. The process involves one protein partner being coated onto a plate surface followed by incubation with the second protein and is used to determine cell viability and cytotoxicity.
Viability dyes
Similar to colorimetric assays, viability dyes are used to determine the proportion of viable to dead cells in a population. Viability dyes commonly used in cell biology and flow cytometry experiments include Propidium Iodide (PI) and Calcein AM. They are used to monitor cell death (necrosis) and distinguish live cells from dead cells.
Flow cytometry
Flow cytometry is a powerful technique used to analyze and quantify different characteristics of individual cells within a heterogeneous cell population. It employs various tools and processes, including emission filters and excitation, to simultaneously measure multiple parameters. This type of high throughput screening contributes to our understanding of cell biology, immunology, cancer research, and many other pharmaceutical and medical fields.
Resazurin assay
Resazurin assays are a type of colorimetric viability assay used to measure cells’ metabolic activity and assess their health and viability. After reconstituting an appropriate buffer or cell culture medium with Resazurin, cells of interest undergo an incubation period to allow them to respond to the treatment. The fluorescence intensity at the end of incubation is proportional to the number of viable cells.
Viability assay: optimizing cell viability during CHO cell freezing
A study was conducted to enhance the cryogenic freezing process of CHO-K1 cell line using a controlled rate liquid nitrogen freezer (RoSS.LN2F) and single-use bags protected by RoSS.KSET. Three freeze runs at different rates in the controlled rate freezer and an uncontrolled run in a static freezer were conducted, assessing post-thaw cell recovery through Trypan blue, LIVE/DEAD, and Fluorescence assays. Results indicate that a freezing rate of −1°C/min is optimal, although other rates can also sustain cell survival to a certain extent. The study underscores the significance of controlled freezing processes over uncontrolled methods, emphasizing the need for standardized protocols in cell-based therapies. For further details, download our study below.
Fact 6: Determining cell viability is time-critical
Above anything else, determining cell viability is a time-critical measure, as cytotoxicity can lead to necrosis, while caspase enzymes may cause apoptosis. Both can lead to the loss of valuable time and money, if not detected in a timely manner, which is why apoptosis assays and other specific assay kits are available. Other factors detrimental to cell health include genetic mutations, immune responses and mechanical stress, as well as hypoxia or cryoconcentration. The latter leads to the formation of ice crystals that can result in the absorbance of water from the surrounding environment, with the following expansion causing damage to cell membranes.
In high-throughput screening including validation for reliability and relevance, timely cell viability assessment is particularly essential in order to ensure valid data generation and interpretation.
Fact 7: Cell viability can be improved at several levels
Improving cell viability requires careful attention to the specific needs of the cell type being cultured. It needs constant control in order to prevent contamination and other detrimental occurrences such as cryoconcentration that can lead to cytotoxicity or apoptosis. This can be achieved at several levels, including the following:
- Contamination control – primarily used to prevent the introduction of foreign contaminants into cell cultures, as those could not only negatively impact cell health but also compromise experimental results. It helps reduce the risk of cytotoxic effects caused by microbial contaminants.
- Cryopreservation – used to preserve cells and tissues at ultra-low temperatures, maintain their characteristics and extend their shelf life. Cryopreservation usually requires a cryopreservation solution with the correct dilution ratio, while the cryogenic freezing itself should be achieved in a controlled manner in order to prevent cryoconcentration.
- Documentation – put in place in order to facilitate traceability and troubleshooting based on detailed records of cell line history, passages, and any deviations.
Cell viability optimization with Single Use Support
The volume of cell-based assays needed for biopharmaceutical manufacturing is increasing at high speed. This results in a need for equipment that is able to optimize cell viability while processing and handling both large and small quantities. Single Use Support has put their focus on the development of dedicated filling and freezing platforms. The solution provider’s aim is to provide the market with straightforward and easy-to-handle solutions for controlled freezing and thawing of cells.
Their plate-based, scalable freeze/thaw unit RoSS.pFTU is the perfect solution for clinical studies and the development of controlled, cGMP-compliant freezing processes. Single Use Support's plate freezers, together with CryoShieldTM – an advanced cryoprotectant technology for cells developed by CryoLogyx – improve the process of preparing cell cultures to carry out cell-based experiments to a large extent.
In addition to the plate freezing platform, Single Use Support have developed RoSS.LN2F as a powerful cryogenic freezer. Based on liquid nitrogen, the innovative solution facilitates controlled freezing for both cell and gene therapies that neither requires direct exposure nor mechanical compressors. This, in turn, ensures a safe, low-maintenance and energy-saving handling, thus promoting an improvement of cell viability.
More about cell viability optimization
A study was conducted to enhance the cryogenic freezing process of CHO-K1 cell line using a controlled rate liquid nitrogen freezer (RoSS.LN2F) and single-use bags protected by RoSS.KSET. Three freeze runs at different rates in the controlled rate freezer and an uncontrolled run in a static freezer were conducted, assessing post-thaw cell recovery through Trypan blue, LIVE/DEAD, and Fluorescence assays. Results indicate that a freezing rate of −1°C/min is optimal, although other rates can also sustain cell survival to a certain extent. The study underscores the significance of controlled freezing processes over uncontrolled methods, emphasizing the need for standardized protocols in cell-based therapies. For further details, refer to the Study Summary.
- Cell Viability, https://www.sciencedirect.com/topics/medicine-and-dentistry/cell-viability, Published 2017