Cryopreservation techniques & best practice freezing methods
Table of contents
ShowCryopreservation involves the preservation of cells, tissues, or other biological constructs by cooling them to extremely low temperatures. The preservation of cells at cryogenic temperature can be achieved using different techniques. This is far from a simple freezing process; it's a carefully controlled method that prevents the formation of ice crystals, thus maintaining the integrity of the biological material.
In this article, we will focus on the various techniques of cryopreservation. Whether a key tool in medical treatments or an essential part of scientific research, understanding the techniques of cryopreservation provides insight into a practice that is as complex as it is vital.
Historical development of cryopreservation techniques
Cryopreservation's historical roots can be traced back to early attempts to preserve biological materials at low temperatures. The initial methods were rudimentary, relying on simple freezing techniques that often led to the formation of damaging ice crystals.
These ice crystals posed a significant obstacle, causing mechanical injuries to cells and tissues. The early practitioners of cryopreservation were limited by the technology of their time, facing challenges such as inconsistent freezing rates and inadequate control over the thawing process.
The transition from early methods to modern advancements in cryopreservation represents a journey marked by technological innovation and scientific discovery. The introduction of controlled rate freezers, cryoprotectant agents, and precise temperature control mechanisms has revolutionized the field of cryogenic freezing. Modern techniques are now able to minimize the damaging effects of ice crystal formation, offering more reliable preservation of various biological constructs.1
Cryopreservation techniques overview
The cryopreservation methods used are tailored to the specific needs of the biological material being preserved. In the following, we'll introduce the different cryopreservation techniques, providing a comprehensive analysis of the methodologies.
Common cryopreservation techniques are:
- Controlled freezing, and
- Vitrification

Vitrification
Vitrification is a transformative cryopreservation technique that rapidly cools biological materials to a glass-like state, bypassing the formation of damaging ice crystals. Vitrification avoids mechanical damage to cellular structures, making it an interesting tool for preserving delicate biological constructs.
The advantage of vitrification lies in its ability to freeze fast with -100°C per minute, keeping the integrity of the biological material. In specific cryopreservation scenarios, vitrification offers a method to ensure that the preserved material retains its functional properties.
Vitrification has the disadvantage that it cannot be applied to all compounds. Vitrification is only an option for certain types of cells, for example not suitable for mammalian cells like CHO cells. It is also a complex process and requires technical expertise. Due to increased complexities in larger volumes, only small volumes are commonly vitrified. Further, the choice of cryoprotectants must be right to avoid inconsistencies and reduced cell viability.2 3
Controlled Freezing
Freezing, also known as "slow freezing" due to the comparatively longer duration is primarily a controlled freezing process. While slow, uncontrolled freezing with static or blast freezing promotes cryoconcentration, it is important to freeze fast and controlled (but still slow compared to vitrification). Recommended is a steady freezing rate around 1-2°C per minute, which can be ensured with plate-freezing methods.
Freezing represents a more controlled approach to cryopreservation. This method allows for controlled ice crystal formation through a gradual cooling process. Freezing typically utilizes programmable freezers that enable precise control over the rate of cooling, thereby managing the ice crystal formation in a way that minimizes potential damage.
This technique has broader applicability and is commonly used for various cell types, including stem cells. The analytical comparison between freezing and vitrification reveals that the choice between these techniques hinges on the specific biological material being preserved. While freezing offers more control, vitrification might be preferable for preserving more elective structures.4
Controlled freezing in cryopreservation: Best practice
Single Use Support focuses on developing innovative solutions for freezing biopharmaceuticals. In the delicate handling of cells, rapid and secure processes take precedence. The freezing specialist’s objectives encompass achieving a high cell recovery rate and ensuring substantial cell viability after thawing.
Two techniques stand out for their efficacy in meeting these goals: cryogenic controlled rate freezing that reaches temperatures as low as -170°C, and plate-based freezing that descends to -80°C, complemented by subsequent storage in liquid nitrogen.5
Cryogenic controlled rate freezing
Cryogenic controlled rate freezing represents a gold standard in cryopreservation. Its main attribute is a controlled cooling rate. By controlling the freezing down to temperatures as low as -170°C, this method minimizes the formation of damaging ice crystals, preserving cell viability after thawing.
Despite the need for advanced equipment, the unmatched control over the freezing process makes it the method of choice for highly sensitive materials. It's a method that emphasizes accuracy, efficiency, and functionality, ensuring that the preserved materials retain their essential biological characteristics.
Single Use Support's RoSS.LN2F is an example of advanced cryopreservation technology. Capable of reaching temperatures down to -170°C/-274°F, this cryogenic liquid nitrogen-based controlled rate freezer is instrumental in maintaining the integrity of sensitive biological samples, such as mammalian cells in cell banking, and offers several benefits:
- Safety and Efficiency: The enclosed platform design mitigates direct exposure, and the absence of mechanical compressors reduces both the complexity of maintenance and energy consumption. These attributes contribute to both safe operation and sustainable practices.
- Precision and Control: With the ability to fully automate and control freezing, the RoSS.LN2F facilitates precise temperature control. Users can customize freeze recipes to suit specific requirements, thereby allowing for optimal preservation conditions.
- Flexibility: Its compatibility with various single-use bioprocess containers, protected by RoSS.KSET, makes it adaptable to different needs and preferences.
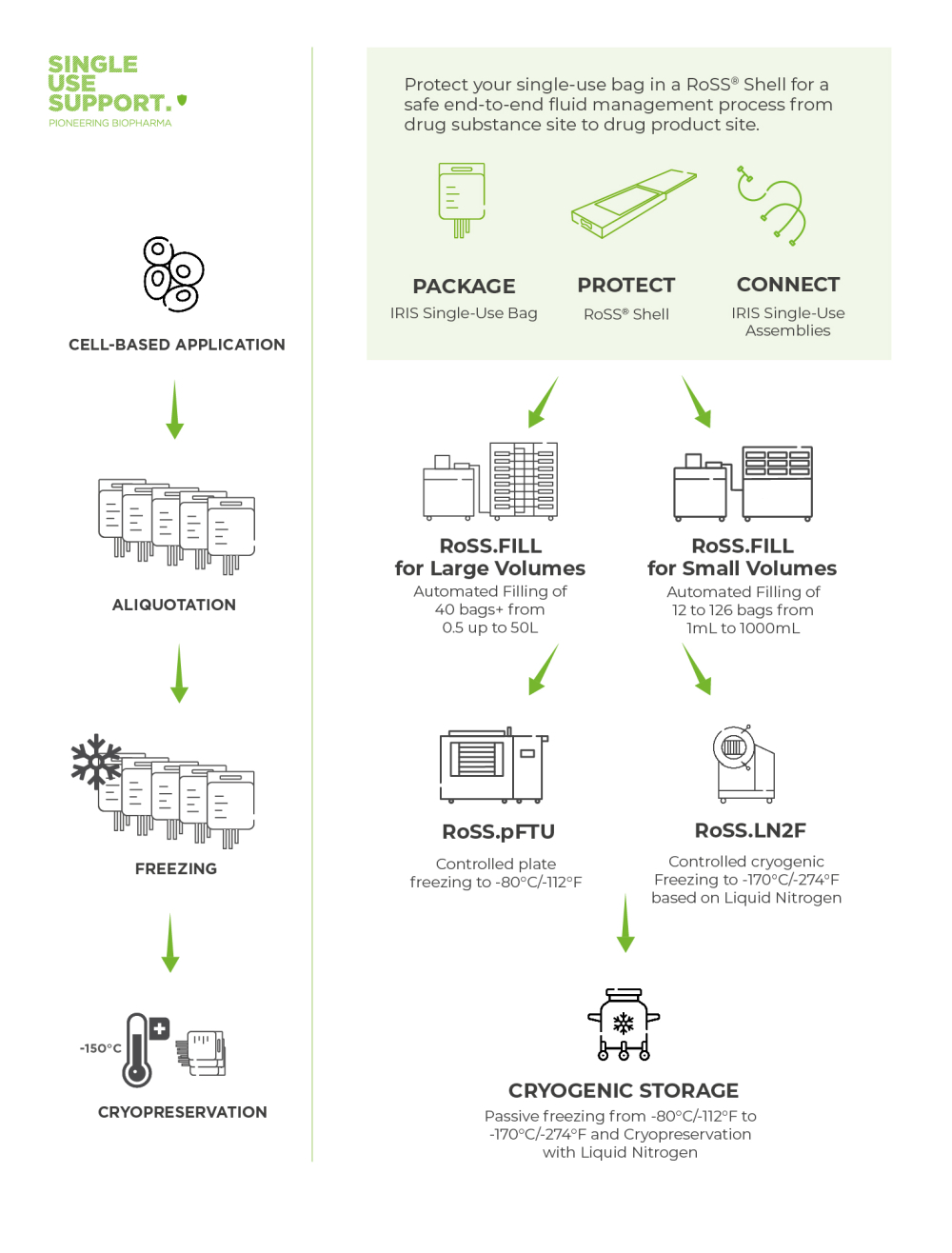
Plate-based freezing
This two-stage process begins with an initial controlled cooling to -80°C / -112°F using specialized freezing plates. This stage ensures uniform cooling, thus maintaining consistency across the sample. Once this temperature is reached, the samples are then transferred to liquid nitrogen storage, where they can be kept stably at temperatures as low as -196°C.
The RoSS.pFTU lab scale by Single Use Support introduces an innovative approach to plate-based freezing, particularly applicable in clinical studies and laboratory environments.
- Controlled Freezing: Capable of achieving setpoint freezing down to -80°C / -112°F, this technology provides a controlled freezing environment. Depending on freezing recipe, the temperature will go down controlled and steady. Phase transition is shortened and cryoconcentration prevented. The system has been engineered to freeze substances with both speed and accuracy, allowing for the preservation of integrity.
- Protection: Utilizing protective single-use shells like ROSS® Shell and the option for small single-use bags RoSS.KSET, the design seeks to mitigate the risks associated with potential compromise during the freezing process. Protection can be provided for bioprocess containers of any manufacturer and size. Product loss is drastically reduced if not eliminated.
Future development in cryopreservation techniques
Emphasizing the importance of accuracy and control, these evolving techniques in cryopreservation aim to minimize damage and maximize viability.
Single Use Support's contributions in this area illustrate the alignment of industry practice with these future trends. The pioneering solutions offer tailored approaches to freezing different biopharmaceuticals, providing secure processes that prioritize high cell recovery rates and substantial cell viability after thawing the cells, notably in cell banking applications.
FAQs about cryopreservation techniques
What are the different methods of cryopreservation?
The different methods of cryopreservation include freezing, vitrification, and some other emerging techniques. These methods enable the preservation of biological material at extremely low temperatures, minimizing ice crystal formation and maintaining cellular integrity.
What is the process of cryopreservation of cells?
Cryopreservation of cells involves cooling cells to extremely low temperatures, typically using freezing methods, to preserve their structure and function. This process often includes the use of cryoprotectant agents to prevent ice crystal formation.
Read more about Cryopreservation
- The History and Principles of Cryopreservation, http://dx.doi.org/10.1055/s-2002-23515, Published 2002-07-26
- Cryopreservation Methods and Frontiers in the Art of Freezing Life in Animal Models, http://dx.doi.org/10.5772/intechopen.101750, Published 2022-01-14
- Principles of Cryopreservation by Vitrification, http://dx.doi.org/10.1007/978-1-4939-2193-5_2, Published 2014-12-01
- Principles of Cryopreservation by Vitrification, http://dx.doi.org/10.1007/978-1-4939-2193-5_2, Published 2014-12-01
- Cryopreservation Basics: Protocols and Best Practices for Freezing Cells, https://www.stemcell.com/cryopreservation-basics-protocols-and-best-practices-for-freezing-cells#:~:text=Cryopreservation%20is%20a%20process%20of,an%20indefinite%20amount%20of%20time., Published 2023-08-11
Products

RoSS.LN2F | Cryogenic Freezer
RoSS.LN2F is a powerful cryogenic controlled rate freezer for temperatures down to -170°C. An enclosed LN2 system and our innovative direct injection system ensure no direct exposure and no mechanical compressors are needed. This ensures a safe, low-maintenance and energy-saving handling.

RoSS.ULTF | Ultra-low Temperature Storage Freezer
RoSS.ULTF is an ultra-low temperature storage fridge for frozen drug substances in different sizes. The ULT storage freezer keeps the desired set point temperature down to -80°C. RoSS.ULTF offers highest storage density, is fully movable and can be modularly adapted to your individual needs.