How to achieve scalable freezing & thawing of biopharmaceuticals
Table of contents
Show"Scalability" is and will remain an important topic in Biopharma.
No matter what area of application, it will be necessary to scale-up or down at some point. Scaling-up from doing research in small lab environments with a handful of scientists to commercial production of bulk drug substances, vaccine production or cell and gene therapies entails a number of challenges.
What is scalability?
Scalability around freezing means the ability to guarantee constant stress on proteins (biopharmaceutical molecules) in all scales, filling volumes and loading scenarios.
How to enable scalability?
Single Use Support fulfils this requirement by standardizing the IFGS – ice front growth speed – for all possible scenarios regardless of the filling volume and type of bags but also regardless of the type of system, i.e. whether LabScale or LargeScale.
The "Scalability Guide" illustrates how to enable scalable results regardless of the
- Size of the single-use bioprocess container
- Size of the freezing & thawing platform
- Number of single use bags you put into the freeze & thaw platforms for a freeze run.
How to achieve scalable freezing & thawing of biopharmaceuticals
Scalability around freezing means the ability to guarantee constant stress on proteins (biopharmaceutical molecules) in all scales, filling volumes and loading scenarios.
Single Use Support fulfils this requirement by standardizing the IFGS – icefront growth speed – for all possible scenarios regardless of the filling volume and type of bags but also regardless of the type of system, i.e. whether LabScale, MidScale or LargeScale.

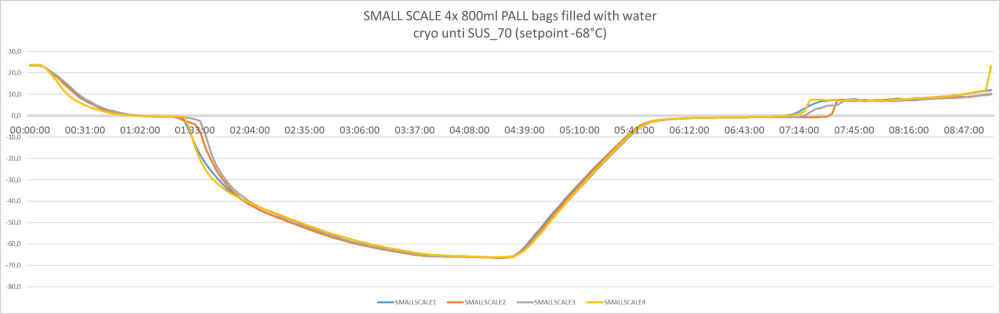
Batch size volume and load independent controlled freezing and thawing results:
Controlled freezing and thawing with all bag sizes – from 3 liters to 300 liters. Example of freeze/thawing curves of a fully scalable process
3 liters / pre-clinical or Phase 1/2
150 liters / Phase 2/3 or commercialized
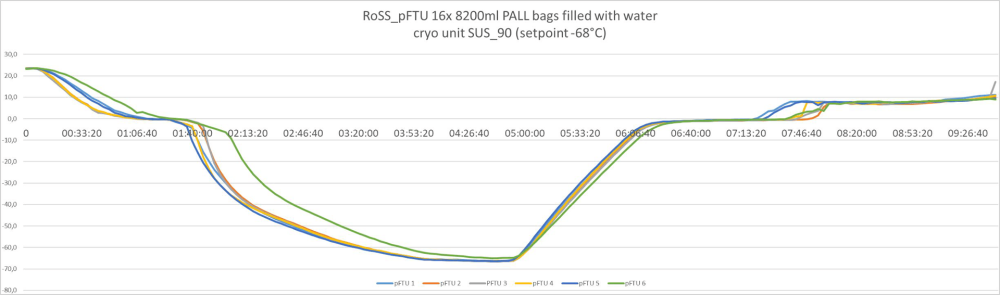
300 liters / commercialized










