Cold Storage Requirements for Active Pharmaceutical Ingredients
Table of contents
ShowActive Pharmaceutical Ingredients (APIs) are the backbone of pharmaceutical formulations, serving as the primary component responsible for the therapeutic effects of medications.
However, many APIs are sensitive to environmental factors such as temperature, light, and humidity, which can compromise their stability and efficacy over time. To maintain the integrity of these vital compounds, cold storage is imperative in cases of temperature sensitivity.
Reasons for Cold Storage of APIs
Some APIs are inherently unstable at room temperature or higher, making them susceptible to degradation. Factors such as chemical structure, formulation, and intended use contribute to the temperature sensitivity of APIs. Cold storage provides a controlled environment where these compounds can remain stable and retain their potency for extended periods.
Impact of Temperature on API Stability and Efficacy
Temperature fluctuations can accelerate chemical reactions within APIs, leading to degradation and loss of efficacy. For example, proteins and biologics are highly sensitive to temperature changes and may denature or lose their activity if not stored correctly. Cold storage helps mitigate these risks by slowing down degradation processes and preserving the molecular structure of APIs.
Cold storage encompasses a range of temperature conditions, each suitable for different types of APIs. Common temperature ranges include refrigeration (2°C to 8°C) cold freezing (down to -40°C), ultra-low temperature freezing (down to -80°C) and cryogenic storage with liquid nitrogen freezing (down to -170°C).
Vaccines: An Exemplar Case
Many vaccines require precise temperature management because of their unique characteristics and makeup. These demands arise from several factors, including how the vaccine is made, its ability to stay effective over time, and its vulnerability to changes in temperature.
For instance, vaccines designed to combat diseases like measles, mumps, and rubella usually remain stable when kept within the range of 2°C to 8°C. Newer types of vaccines, such as mRNA vaccines, which contain large molecules, are more delicate and require storage at ultra-low temperatures, sometimes below -15°C or even as low as -70°C.1
An Overview of Cold Storage Options for APIs
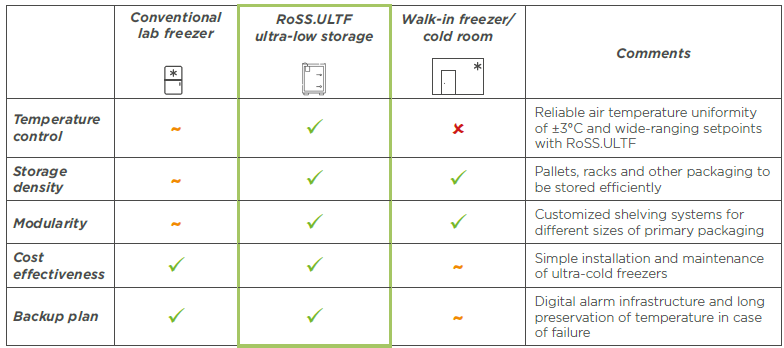
When considering cold storage options for active pharmaceutical ingredients, there is a wide array of choices available, each with its own advantages and limitations. Traditional methods such as cold storage rooms and upright or chest freezers have been widely used in the industry for their cost-effectiveness and ease of use.
However, these conventional technologies are not without their drawbacks. They struggle with precise temperature control, along with issues like limited storage capacities, compatibility problems with primary packaging, and the need for manual loading, all of which can impact final product quality and hinder efficiency and flexibility in manufacturing facilities.
In response to these challenges, Single Use Support offers innovative solutions like RoSS.ULTF, which aims to combine the best features of ultra-low temperature storage technologies. RoSS.ULTF boasts several advantages, including superior storage density, modular shelving systems for convenient loading, precise temperature management down to -80°C, and compatibility with various primary packaging formats.2
Ultra-cold Freezing & Storing of APIs: Best practices
Single Use Support's solutions offer a robust answer to the demands in ultra-cold freezing and storing of active pharmaceutical ingredients. Ultra-cold storage freezer RoSS.ULTF provides high-density ultra-cold storage, boasting precise temperature control capabilities that enable temperatures as low as -80°C.
Freezing Active Pharmaceutical Ingredients
Prior to storing at ultra-cold temperatures, it is necessary to undergo the process of freezing. Freezing down APIs using plate-based freezing platforms, such as the RoSS.pFTU system from Single Use Support, provides several advantages. Traditional cooling methods, such as static freezers, often face challenges with volume-dependent and hence inflexible freezing rates, leading to inefficient heat transfer and potential damage to complex proteins. Plate-based controlled freeze and thaw platforms, on the other hand, offer precise control over freezing rates, maintaining homogeneity throughout the active pharmaceutical ingredient.
Read more: Freezing drug substance
Single Use Support has developed automated end-to-end solutions specifically for the aseptic management of fluids and for controlling freeze and thaw cycles of APIs. These systems are designed to seamlessly integrate with other brands, and Single Use Support has also introduced additional products to streamline the entire process from start to finish.
For instance, the RoSS® Shell is engineered to ensure the robustness and protection of frozen drug substances contained within single-use bags. These bags can be filled with minimized risk of product loss using automated aseptic filling systems.
Ultra-cold Storage of APIs
After freezing down the drug substances in single-use bags, they can be stored in ultra-cold storage solutions like RoSS.ULTF. It offers versatility to meet various cold chain storage needs. Its modular interior allows for scalability, stackability, and high storage density, making it adaptable to processes ranging from lab-scale studies to bulk production and storage of biopharmaceuticals of different primary packagings.
RoSS.ULTF offers several advantages over conventional storage solutions. It provides reliable air temperature uniformity, ensuring consistent and stable storage conditions for active pharmaceutical ingredients. Additionally, RoSS.ULTF features GMP-compliant digital alarm management, promptly alerting operators to temperature deviations.
Temperature monitoring
Temperature monitoring is critical in maintaining the cold chain, and RoSS.ULTF offers comprehensive monitoring capabilities. Continuous monitoring systems provide real-time vigilance, alerting operators to any deviations from the desired temperature range for proactive intervention.
RoSS.ULTF automatically monitors temperature and provides automated reports for easy viewing. Manufacturing execution systems (MES) allow for the monitoring and control of storage conditions from a distance, enhancing overall system efficiency and ensuring the integrity of the cold chain throughout the API manufacturing process.
Conclusion
In conclusion, the stringent cold storage requirements for active pharmaceutical ingredients underscore the critical importance of precise temperature control to maintain their stability and efficacy. Traditional methods, while widely used, often struggle with limitations such as imprecise temperature control and manual loading requirements.
Innovative solutions like RoSS.ULTF from Single Use Support offer a promising alternative, providing high-density ultra-cold storage with precise temperature management capabilities down to -80°C. These advancements not only ensure the integrity of APIs but also enhance efficiency and flexibility in the manufacturing process.
- Ultra-low-temperature (ULT) storage of vaccines, https://www.susupport.com/knowledge/vaccines/ultra-temperature-storage-vaccines, Published 12.2023
- Best choice for cold storage of Biopharmaceuticals - A Comparison, https://www.susupport.com/knowledge/freeze-thaw/best-choice-cold-storage-biopharmaceuticals-comparison?rel=search, Published 01.2023











