Best choice for cold storage of Biopharmaceuticals - A Comparison
Table of contents
ShowThe number of cold storage freezers for the biopharmaceutical market is countless.
Some neither fulfill requirements of Big Pharma nor preferences for small biotechs. If you seek for a comparison in the myriad of options and promises with regards to ultra-low temperature cold storage freezers, the following will be interesting for you.
What are the biggest constraints with conventional cold storage technologies?
Conventional technologies in Biopharma for cold storage of any drug substance are either cold storage rooms and upright or chest freezers. The latter have more similarities with common refrigerators holding sub-zero temperatures with optional setpoint temperatures. Both technologies are well-established for a reason as they offer options to store biopharmaceuticals either in a very inexpensive way or they have simple and hands-on solutions for quick installation and fixes.
However, there are some constraints at manufacturing sites, such as:
- Inefficient storage of drug substance whilst having limited storage capacities
- Incompatibilites of primary packagings
- Being dependent on manual loading due to inflexibility of systems
- Lack of holding ultra-low temperatures down to -80°C
- Long lead times

RoSS.ULTF: Merging the best of ultra-low temperature storage technologies
Single Use Support’s innovation RoSS.ULTF offers superior attributes for long-term cold storage of biopharmaceuticals. It is embedded into an end-to-end process solution for bulk drug substance. Yet it can be applied universally to increase efficiency for storing all primary packagings.
- Highest storage density: In case of limited storage room on-site the most convenient way out of scarcity of storage room is to increase storage density. With RoSS.ULTF, multiple single use bags protected in RoSS can be stacked or inserted into different shelving system. Also, one pallet can be placed inside RoSS.ULTF. Upon request, RoSS.ULTF could be stacked itself one over another giving the option to double storage density.
- Modular shelving system: Single-use bags and rigid bottle container or carboys can be loaded into RoSS.ULTF either in adjustable shelves. Alternatively, fully loaded Trolleys or Racks may be loaded into the cold storage device in order to avoid manual loading which in turn decreases staff work force, risk of breakages and relieving health of staff.
Read the article Primary and Secondary Packaging of drug substances to learn more about safe packaging solutions.

- Controlled Temperature: Long-term freezers for cold storage are not constructed to freeze drug substance from ambient temperature to ultra cold temperatures at approx. -80°C. Fast freezing, such as with RoSS.pFTU is proven to be best to grant product stability of drug substances. Long-term freezers are built to hold the cold temperature. With smallest air temperature uniformity, smart insulation technology and a wide range of setpoint temperature RoSS.ULTF convinces with its performance - ultra cold storage is the solution. Qualification and validation of freezing units grant for reproducibility and fostered reliability.
- Lower TOC: The price tag itself does not reveal the total cost of ownership. Especially when it comes to long-term cold storage, the operational expenses, process efficiency and required infrastructures outweigh the initial costs. When looking at a time of usage over few years, the costs are comparable among all technologies.
- Compatibility: Especially when using multiple primary packagings it is difficult to find one solution that fits to them all. High storage density paired with a modular shelving system gives room for all primary packagings, such as single use bags, carboys or cryovault.
- Part of end-to-end solution: RoSS.ULTF is one part of end-to-end process solutions from Single Use Support. It therefore grants seamless transitions among technologies for freezing, thawing and shipping. However, it is a platform completely independent on vendors and sizes of single use bags and bottles.
- Favorable features: RoSS.ULTF can be installed nearly everywhere - provided that there a network connection for power supply. With its safety features as door ajar alarm, lockable front cover and optional temperatur mapping it supports both staff and the frozen products.
Superior attributes for long-term cold storage with RoSS.ULTF
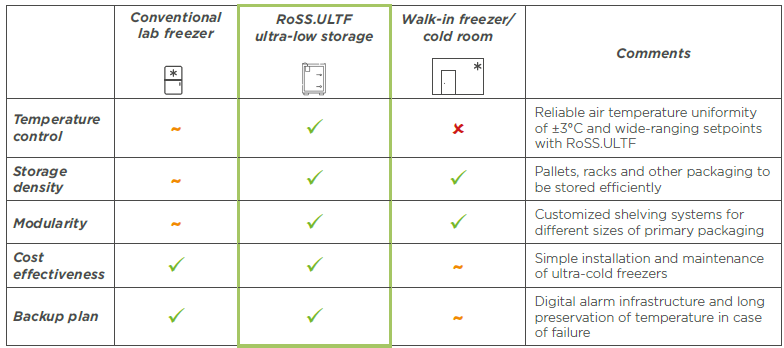
Merging the best of cold storage technologies, RoSS.ULTF outperforms - as illustrated in the comparison table below.