Frozen Storage and Shipping in biopharma with RoSS
Table of contents
ShowFrozen storage and shipping of drug substances is indispensable in the biopharma industry. Nevertheless, drug substances are precious and sensitive, not only requiring careful handling but also reliable protection throughout the entire logistics process. In order to allow for both, the liquid substances are frozen for shipping and storage in specialized containers that keep them safe in a temperature-controlled environment throughout the journey.
Single Use Support’s RoSS® Shell (robust shell system for frozen storage and shipping), composed of an outer sleeve of robust stainless-steel and an adaptive inner layer of 3D foam, contributes to a safe supply chain and timely deliveries of drug substances. It warrants the single-use bag’s intact arrival at its final destination, keeping its precious and perishable contents protected from biocontamination and other damages.
The highly flexible and scalable nature of the single-use components used for the construction of RoSS makes handling very easy and user-friendly, while acquisition and storage costs as well as space requirements can be reduced significantly. As a result, cold chain transportation processes based on advanced single-use technologies offer reliable, safe and controlled handling of biopharmaceutical liquids, which are not only highly valuable but also very sensitive.

Flexible, versatile and user-friendly solution for frozen storage and shipment
With the varying requirements of the biopharmaceutical industry, of labs and manufacturers in mind, RoSS has been designed to offer flexible, versatile and user-friendly protection for single-use bags of any size and brand. It is both bag-agnostic and scalable, protecting filled bags of any given manufacturer and volume. Furthermore, RoSS is extremely space-efficient, reducing costs for storage facilities and transportation services like shippers and other third-party logistics providers. Also, the storage and shipping solution allows for easy handling by staff, distributors, and warehousing teams for temperature-controlled storage.
Read more about Storing and shipping frozen drug substance - why single-use systems are the optimal solution
Protection of bulk drug substances in single-use bags
The outer shell: Robust stainless steel
Thanks to its thermal diffusivity, the stainless-steel used for the outer shell is not only acting as a protective buffer but also offers the best possible temperature transfer from freezer to bag to liquid. As a result of the direct contact between the plate freezer and the stainless steel plates covering the container, the substance contained in RoSS® is evenly frozen. The close contact between freezing plates and liquid substance significantly speeds up the process by facilitating a direct transfer of the cold.
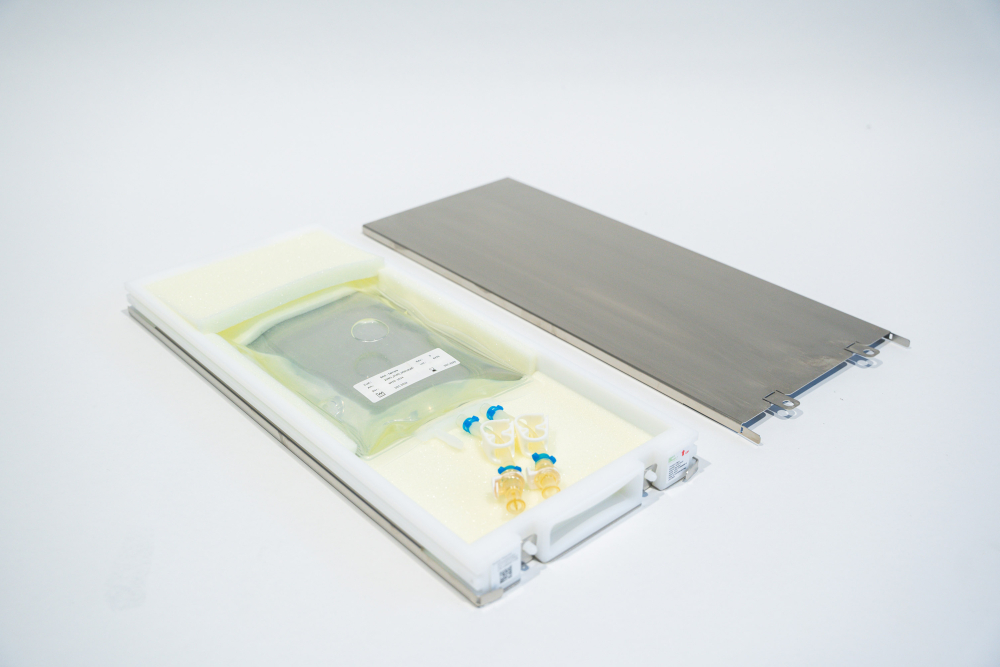
The interior: 3D foam for added protection during cold storage and shipping
While the outer shell is made of robust stainless steel, specialized foam on the inside adds further stabilization. Single-use bag and content are shielded by a layer of soft 3D foam that adapts to the bag’s shape during the freezing process, eventually fully immobilizing it. The design leaves space for tubings to make handling a breeze.

In short: Taking inspiration from an oyster, RoSS combines stainless steel on the outside and protective foam on the inside to make for the perfect robust and compact protection of your valuable goods during shipping and storage:
- RoSS is independent from any single-use bag manufacturer, protecting what’s valuable in your single-use bag of choice.
- RoSS is scalable to different volumes to cater for both lab and production environments.
- RoSS ist the scalable as flexible choice for frozen storage and shipping single-use bags (of different suppliers) in biopharma
As an added benefit, there is no ambient air that requires cooling, as is the case when utilizing traditional freezers. The homogenous outcome when employing plate freezers not only maintains the substance’s original quality but also protects it from potential damages during shipping and thawing of drug substance.
The shell is tamper-proof so the bag contained within cannot be accessed during the entire controlled freezing and logistics process.
Freeze-Thaw Platform for frozen storage and shipping of drug substances
While RoSS shells are ideal when it comes to the overall protection of frozen drug substances, they form only one part of the state-of-the-art RoSS freeze thaw system, which in itself offers a number of benefits for pharmaceutical labs and production sites. As a main selling point, it helps to avoid human failure in the handling of drug substances as it offers a process that is fully controlled and monitored from beginning to end. In addition, it can be adapted and scaled to meet any requirements and needs, thanks to the utilization of single-use components. Like this, the RoSS freeze thaw platform furthermore
- significantly reduces the need for cleaning and sterilization
- minimizes production costs
- maximizes production output
- is easy and simple to dispose of
- reduces power and water consumption
- minimum storage space
- optimized for frozen storage and cold chain shipping
Simply put: Using RoSS means optimizing your process by significantly reducing risks and the potential for human error.
FAQs about Frozen Storage and Shipment
Can RoSS shells be recycled?
Yes, RoSS shells can be recycled. Customers are encouraged to get in contact with their local waste management. Alternatively, RoSS shells can be sent back to us at Single Use Support for proper disposal.
What is needed for cold and frozen storage and shipping?
It is hard to determine what exactly is required in frozen storage and shipping, as this depends on the specific product. While certain products are cooled by gel packs or the refrigerated interior of a truck (e. g. when an entire truckload of pallets with food are to be transported), certain drug substances need to be stored at lower temperature ranges. This can be achieved by the means of dry ice, but also via dedicated cold storage freezers, providing real-time temperature monitoring and high consistency in temperature.
What is primary and secondary packaging?
The primary packaging is the packaging that comes into direct contact with the pharmaceutical products. A secondary packaging is the outer packaging, which provides extra protection to the primary packaging during storage and transportation to reduce the risk of breakages of the primary packaging. Read more in the article: primary and secondary packaging for drug substances









