Shedding light on optimal freezing results for drug substance
Table of contents
ShowHow x-ray views affirm plate-based freezers as spearheaded freezing technology in Biopharma
One of the most critical process steps for biopharmaceuticals is freezing the product in single use bags to sub-zero. No matter if companies are freezing bulk drug substance, mABs or vaccines after the downstream manufacturing process or for cell & gene therapies – either as supplement or as industrialized remedy for patients – freezing causes stress for the product. Therefore, it is of great importance to secure its product quality with advanced freezing technologies.
Once a controlled and scalable freezing process is established, it is possible to be reproduced for both, a small-scale model but also for an industrialized scaled-up manufacturing process.

Design of experiment (DoE) to evaluate freezing technologies
Single Use Support wanted to take a closer look into the single use bags when they’re at frozen state. The aim was to visually compare the interfacial stress of frozen single use bags: How does the bag experience stress in two different approaches of freezing, plate-based freezers and conventional static freezers?
Single Use Support has been made available a computer tomography scan to x-ray both identical single use bags with the only difference of the freezing technology. One bag has been frozen in a plate-based freezer, the other in a static freezer.
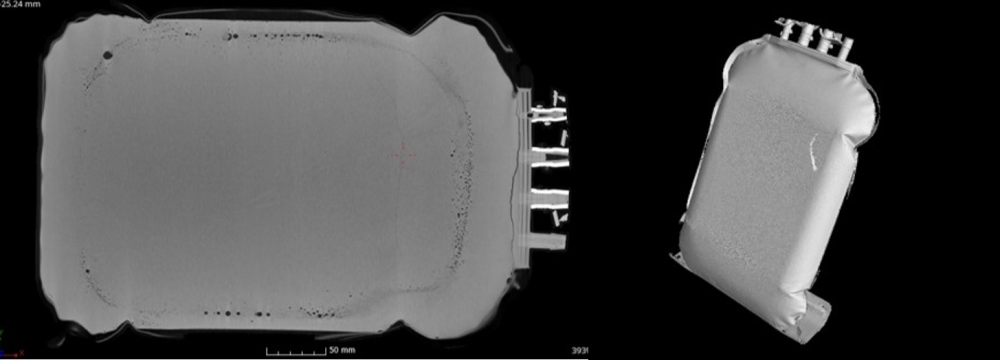
Cross checking quality of frozen bags
Scanning the cross sections of both bags, it is possible to see a thin line in the middle of the single use bag, which is the LPF, the “last point of freeze”. As the ice fronts grow from top and bottom, they will meet – in best case – in the middle.
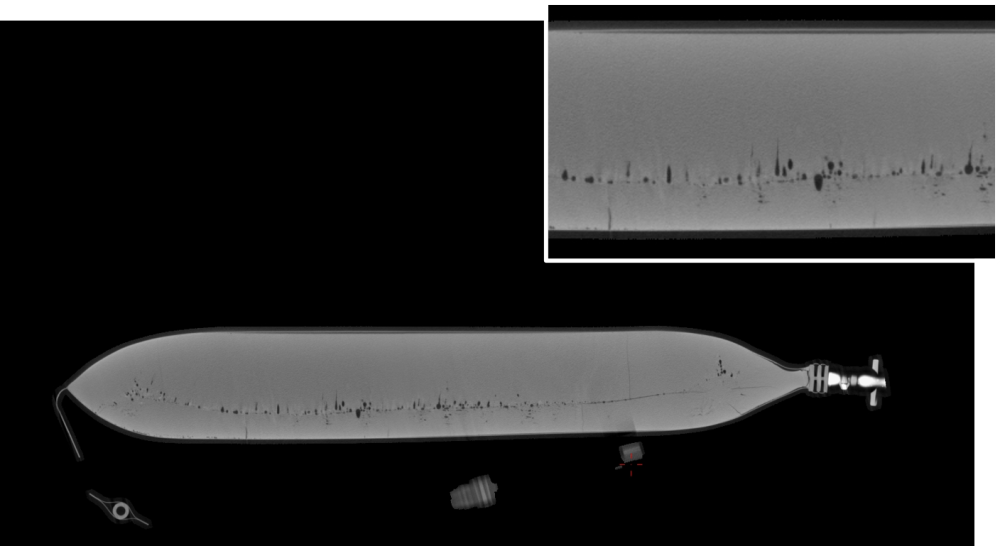
How is freezing in a Static Freezer?
- The line of LPF is bent downwards. What does this mean? It shows that the ice front grows faster at the top than at the bottom. Since the bag lies on a shelf during the freezing process the cold air mostly enters the bag from the top. This leads to a curved uncontrolled ice front growth.
- There are clear marks for higher/lower density visible that accompany the LPF. This effect is called cryoconcentration. Reasoning with slow freezing, the drug substance, its proteins, and its other components detach from water and gather in the center of the bag as the temperature is falling. So, water is being frozen at the bag surface at first and all the highly valuable drug substances at last, leading to increased pressure and denaturation of the product. In the end, ice crystals are distributed inhomogeneously throughout the bag.
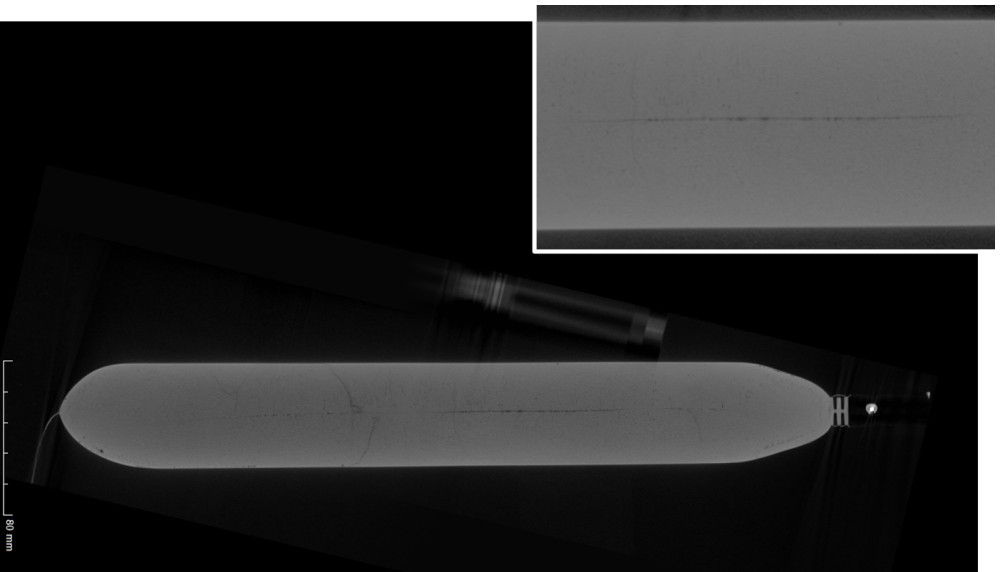
And how is freezing in a Plate-based Freezer?
- The line of LPF in the center of the bag is straight and thin. The straight line confirms that the cold enters the bag from top and bottom at constant fast speed. Underlined by the homogeneous density shown in the picture below the fusion of both ice fronts from top and bottom has been very controlled and predictable.
- There is one unified color and microstructure from top to bottom. The homogeneity of the frozen drug substance in the single use bag proves once more that constant fast freezing through a plate freezer for biopharma or small lab freezer with controlled ice front growth allows crystals to distribute homogeneously in single use bags. There is no cryoconcentration. The outcome: more protein activity and better freezing outcomes. This comes along with the proven outcome of various research & development groups.(1)
Summary of comparison
Advocating plate-based freezing technologies in the Biopharma industry, Single Use Support is at the forefront of innovating drug substance management during logistics for single use technologies providing best freezing outcomes. The portfolio of products ranges from filling, freezing, cold storage, shipping, thawing to draining of biopharmaceuticals into primary packages, such as single use bag, rigid containers (bottles) and vials. Unprecedented end-to-end solutions for handling of biopharmaceuticals are built upon plate-based freezing-thawing of RoSS shells as secondary packagings.
Static freezers are constructed to hold setpoint temperatures and may be suitable for cold storage of already frozen single use bags. However static freezers are limited in freezing down liquids from ambient condition to sub-zero temperatures. Freezing time from ambient temperatures to -60°C takes at least 25 hours.
Only with plate freezers it is possible to achieve a fast, controlled and homogeneous freezing behavior which remains reproducible and predictive for your drug product.
If you are interested in this topic, please get in contact with us. We are open to collaborate as we aim to educate ourselves further in order to achieve optimal freezing results.
(1) Kolhe, P., Badkar, A.: Protein and solute distribution in drug substance containers during frozen storage and post-thawing: a tool to understand and define freezing-thawing parameters in biotechnology process development. 2011. Available at: https://pubmed.ncbi.nlm.nih.gov/21302371/










