At the forefront of Pharma 4.0
Table of contents
ShowFirst achievements of digitalization in the pharma industry
All along the journey of an API (active pharmaceutical ingredient) from research & development to production and distribution, Pharma 4.0 will contribute to a process optimization to a yet unknown extent. Digitalization of products and processes in an industrial setting will be the new standard since it offers advantages in establishing sustainable standards and in improving the performance in operative handling in general.
What’s already in play?
There is no full Pharma 4.0 manufacturing in place yet, but there are first innovations to build on.
There are currently numerous manual handling steps in biopharmaceutical manufacturing coming along with a high degree of failures in manufacturing facilities deriving from human errors in operative handling. The numbers range from 46%[1] to 80%[2] to be human errors of all operative errors. Therefore automating process steps which forms the basis for implementation of Pharma 4.0, gives hope for a major decrease of failures due to human error.
Examples for automating manual operative handlings are:
Automated filling of single-use bags and bottles
After inserting single-use manifolds manually, liquids are filled into single-use bags, bottles in different scales in a fully automated manner with RoSS.FILL. Assisted by highly accurate weighing scales it is possible to report the filling volume per bag to the MES system, be it Siemens, Emerson DeltaV or other. The gathered information will not only be stored in the software of the single-use technology, but also be attached as barcode or RFID tag on the individual filled primary packaging.

Automated sealing off single-use bags
After successfully dispensing liquids into single-use bags or bottles, it is possible to seal off the single-use bags from the fluid path manifold in a fully automated manner. Integrated in the valves of RoSS.FILL the heat-inducted separation enables aseptic sealing of attached single-use bags from the manifold within a short space of time. Especially in cell & gene therapies when using multiple small single-use bags such an increase of efficiency in sealing saves time and costs whilst securing a standardized and safe operation.

Homogenizing and cooling single-use bags
RoSS.PADL is a scalable platform for cooling single use bags whilst using two paddles that gently massage the bag to ensure that there is a homogenous mixture of the solution. Replacing human intervention to massage the bags during draining, RoSS.PADL drives automation of this still often very inefficient process step. Multiple RoSS.PADL’s can be controlled by a single control unit or be controlled from a RoSS.FILL unit.

Shaking functions
Constant movement of biopharmaceuticals is necessary to regain homogeneity and protein quality in some occasions, for example during thawing. RoSS.SHAK as standalone shaker or RoSS.pFTU Freeze Thaw Platform with integrated shaking functions support standardized homogeneity of drug substances in single-use bags and bottles.
Examples of Digitalization of manufacturing processes from Single Use Support are as follows

Product Digitalization through RFID & smart shipping
Radio-frequency identification tags – short: RFID tags – can be attached to all physical products which are part in the manufacturing process. It enables digitalization of each product which is in touch with your single-use bag. It is therefore the entry ticket to the Internet of Things (IoT). Various data can be fed to the IoT and stored on a cloud-based data hosting. Different products can thus connect, interact and also learn through AI-driven parameters.
RFID tags can be placed anywhere: on the single-use assembly, on your single-use bag, on the bag protecting RoSS shell, or on your shipping container RoSS.SHIP. Its connectivity allows data to be interacting between platform systems and sterile consumables. The manifold, for example, has a unique RFID tag which is read and stored in the RoSS.FILL so that it records which manifold was used to fill each bag.
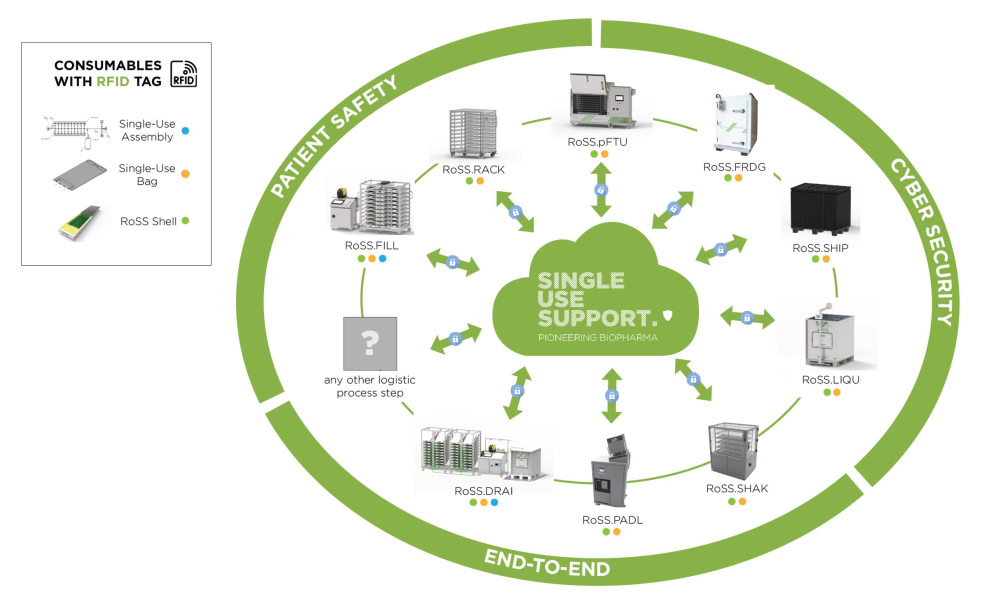
Data can be the filling volume or speed of the single-use bags and can be derived from software reports from the automated filling platform RoSS.FILL. It can also hold freezing curves from manually or recipe-driven freezing and thawing performance through automated freezing thawing platform RoSS.pFTU. Even when your product is outside manufacturing sites, it is possible to view data transparently and in real-time: With the already well-established “smart shipping” of biopharmaceuticals. Preferred data such as temperature, g-force, humidity and much more will be collected and documented during international transport. Data from transportation or anything via RFID will be hosted in a web-based cloud.
In a nutshell: An RFID tag that is attached to the RoSS shell is a closed system throughout the entire manufacturing process and enables a seamless full lifecycle tracking of your product alongside the bioprocessing end-to-end process. Putting this RoSS shell into the RoSS Smart Shipper allows realtime tracking of the drug substances.
Cloud-Based Solutions
All collected data from different RFID tags and smart shippers can be hosted in a centralized web-based cloud from Single Use Support. Fully accessible to the client but with the highest cyber security, it is possible for Biotechs and Biopharma manufacturers to monitor and control data. This provides an overview of the entire manufacturing process in different scenarios, for example when having production outsourced to CMOs or CDMOs, but also of in-house manufacturing which is often divided into detached departments.
Most importantly data science helps to recognize and mend weak spots in the manufacturing process and to establish a sustainable continuous manufacturing through full process automation. Optimization might be achieved by managing creation of recipes, gathering and comparing reports or providing an overview on due dates of services.
Virtual Services through Augmented Reality
Digital services round off the capabilities of Single Use Support:
Planned or unplanned after-sales services of single-use platforms can be performed with virtual glasses. The implementation of augmented reality in service has achieved a 70% success rate to solve inquiries so far. The possibility for operators to have access to immediate and comprehensive support has therefore decreased downtime of production significantly.

For any other support to provide information on Single Use Support’s products, it is possible to request a guided virtual product demo.

Advantages in pioneering Pharma manufacturing
Early adopters of Pharma 4.0 gain expertise and improve the standard of their entire pharmaceutical manufacturing process instantly. It opens new doors for future process optimization by establishing full automation in biomanufacturing through IoT.
This in turn offers the advantages to:
- Avoid human errors: Performance will be improved radically in operative handling
- Establish standards: Manual intervention of simple tasks, such as homogenizing single-use bags or sealing off single-use bags become void.
- Improving process: Upon collected data it is faster, more transparent, and more profoundly to turn the adjusting screws to enhance effectiveness and efficiency of manufacturing.
- Full control over data: Full data integrity provides insights in monitoring & tracking of different product batches in real-time. Full transparency facilitates efficient documentation and ramps up efficiency in bioprocessing. Moreover, another goal is to identify ways to improve product quality throughout manufacturing to secure optimal quality for patients.
If you are interested to explore Pharma 4.0 in all its beauty, please get in contact with us. We are open to collaborate as we aim to push the boundaries in order to achieve optimal Biopharma process solutions.
- Aspen Media Inc.: Survey, 2020: https://www.susupport.com/reduce-human-error-biopharma/
- Pharmaceutical Online: Article, 2016: Reducing Human Error in Pharmaceutical Manufacturing. https://www.pharmaceuticalonline.com/doc/reducing-human-error-in-pharmaceutical-manufacturing-0001










