Start Pharma 4.0: Examples to Advance Ultra-Cold Storage of Drug Substances
Table of contents
ShowPharma 4.0 is no longer a thing of the future. Industry 4.0 for biopharmaceutical manufacturing is here now.
Innovative manufacturers are striving for operational excellence in as many areas of bioprocessing as possible. In return, they will benefit from standardized and fully automated processes that reduce the number of unpredictable events, product loss, cross-contamination, and resource consumption. Successful adoption of Industry 4.0 will therefore contribute to cost efficiency, patient safety, and sustainability in pharmaceutical manufacturing.
This paradigm shift isn't just about automation; it's about orchestrating a symphony of smart devices and systems to create seamless, efficient operations across the biopharmaceutical landscape. A prime example of this shift is the integration of cold drug storage into the Pharma 4.0 framework. Interest in advancing this process step is already high among pharmaceutical companies. What does a smart ultra-cold storage process look like according to Pharma 4.0 principles? Let’s take a closer look at ways to automate ultra-cold storage through Single Use Support.
Opening Doors for Pharma 4.0
Traditionally, freezers and cold chain drug storage facilities are managed manually, requiring human intervention for tasks as simple as opening doors. Imagine a storekeeper leaving a door open for too long, disrupting the delicate ecosystem inside and allowing ice crystals to grow on the door, which can ultimately reduce the freezer’s insulation.

Autonomous forklifts navigate the facility to load and retrieve materials to and from ultra-low temperature (ULT) freezers. But the real magic lies in the interplay between technology and precision. The freezer doors need to open at just the right time – not too long to avoid temperature loss and ice formation. This choreography of control is achieved through robust software integration that links the storage freezer to the process control system.
But that's not all. Communication between the robot and the ultra-cold storage freezer is critical for flawless material handling. The robot must position itself precisely for loading and unloading operations. This is where the power of Pharma 4.0 comes in – ensuring that this dance is executed to perfection. Every step, every access is documented by comprehensive audit trails that are securely stored in the central process control system.
The interconnection of Freeze-Thaw Units (FTUs) and ULT storage freezers with autonomous forklifts is a vivid example of Pharma 4.0 perfection. The ability to implement automated door systems triggered by the process control system and to store substances at a chilling -75°C while maintaining stringent alarm systems ensures that drug substances are preserved at their best. The result? A seamless, controlled environment for drug substance storage.

Robotics Takes Over Cold Chain Management
Integrating robotics into ultra-low temperature (ULT) storage isn’t just an evolution; it's a leap toward greater efficiency, safety, and precision in cold chain management and storage of drug substances. It can help improve cohesive process steps.
- Automated loading & unloading: Automation eliminates the variability associated with human intervention, ensuring consistent and safe operations.
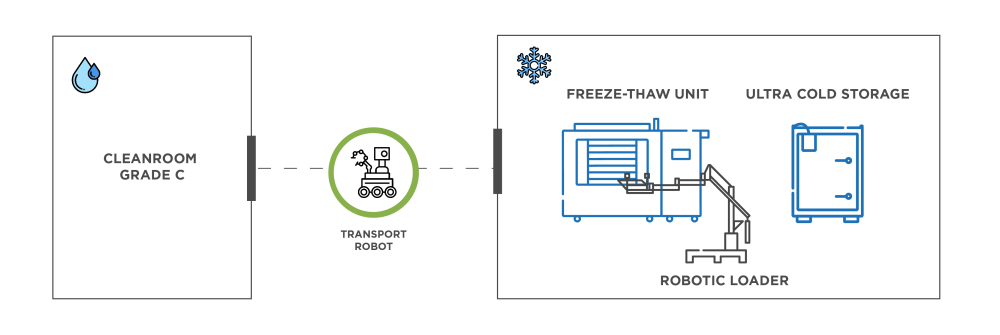
- Transportation: The movement of drug substances from a Grade C clean room to freezing and storage platforms can be orchestrated by transport robots. Autonomous forklifts can also safely pass through airlocks. They help navigate the facility and ensure materials reach their destination without compromising sterility and integrity.
- Loading into plate-based freezers: One robot can load two plate-based freeze-thaw platforms, such as the RoSS.pFTU Large Scale, with a total of up to 800L in single-use bags, protected by secondary packaging shells. The smart layout of robotic systems minimizes congestion and optimizes drug placement and retrieval.
- Transit of frozen liquids to ultra-cold storage: The conveyor becomes a gateway for transporting frozen bioprocess containers in RoSS® shells toward the ULT freezers.
- Loading into ultra-low temperature freezers: Robotic loaders place RoSS® shells into ultra-cold storage freezers, such as RoSS.FRDG – and retrieve them for cold chain shipping.
The concept of an intelligent, integrated end-to-end cold chain process solution is becoming more than just a notion. By harnessing automated technologies, biopharmaceutical manufacturers can achieve precision, efficiency, and safety.
Reasons to Start Now
Embracing Pharma 4.0 is about more than just adopting technology; it's about reimagining processes. The marriage of cold storage and Pharma 4.0 isn't merely about opening doors; it's about opening doors to a future where biopharmaceutical manufacturing reaches new heights of efficiency, reliability, and precision.

The marriage of cold storage and Pharma 4.0 isn't merely about opening doors; it's about opening doors to a future where biopharmaceutical manufacturing reaches new heights of efficiency, reliability, and precision.
Alexander FuchsPredictive maintenance is another emerging area that can be part of integrated fluid and cold chain manufacturing solutions. By continuously monitoring any changes, caused by pressure, temperature or else, technologies can report and flag deviations. Picture a stepper and sealer valve used in aseptic aliquoting with RoSS.FILL alerting the process control system when action needs to be taken, such as integrity testing or replacement.
With predictive maintenance as part of Pharma 4.0, the orchestrated operations eliminate the risk of temperature fluctuations, ensuring that drug substances remain intact and viable. In addition, automation saves time and minimizes human error, allowing researchers to focus on tasks that truly require their expertise.
As we stand on the brink of this transformation, it's clear that integrating robotics into ULT storage is not just about machines; it's about advancing standards, pushing boundaries, and creating a future where the cold chain management continues to evolve.








