Industry 4.0 in Biomanufacturing: RoSS.KSET as an example
Table of contents
ShowIn many branches and sectors, Industry 4.0 has long gone from being a buzzword to reality: In Pharma 4.0 intelligent industrial applications and IoT enable players in the bioeconomy industry - an umbrella term for biomanufacturing and biotechnology - to transition to operations that help them improve their productivity. Pharma 4.0 also has become a major part in single-use technologies, as it brings companies to the next level concerning the scalability, productivity and efficiency of their processes and products.
This is made possible by two key factors, one being the information-driven automation of both existing and newly developed infrastructure. In addition, biotech and biopharmaceutical companies can benefit from the introduction of advanced, state-of-the art monitoring and control strategies that cover the entire developing, manufacturing and drug substance logistics process.
Industry 4.0 in Biomanufacturing
Biomanufacturing per se is defined as an industrial production using scalable, validated processes that utilize microorganisms and biological systems like animal cells, plant cells or stem cells to develop and produce biomolecules and -materials to use for therapeutics. So while the aim of biomanufacturing companies is to improve the quality of life of humans with the development of ever-new products - from stem cell therapies to genome sequencing and therapeutics - all of which are based on organic microbes, artificial intelligence plays another important role.
Industry 4.0, also known as the fourth industrial revolution, which the biotechnology and bioprocessing industries are currently experiencing as Pharma 4.0, goes to prove the point: The integration of IoT in synthetic biology, is not a new reality. Many biomanufacturing processes have been subjected to digital transformation for years, resulting in improved unit operations as well as an overall favorable track record, while obviously complying with both regulatory requirements and GMP standards. This also goes for single-use technologies, which aim at making supply chains more efficient and reduce product loss by providing specific protective technologies, equipped with Pharma 4.0 mechanisms.
How does Industry 4.0 revolutionize Biomanufacturing?
The bioeconomy industry profits from Industry 4.0 initiatives in a number of different ways. Digital intelligence and connectivity can be embedded in industrial applications to streamline processes and advance automation.
The examples range from development and bioprocessing (for instance in genetic engineering, metabolic engineering or by automating cell culture manipulation or genome sequencing) to enabling faster and more reliable supply chain processes in single-use technologies. New developments allow for tracing and tracking of any product or component in real-time, which is particularly important when it comes to handling sensitive synthetic biology products. Moreover, Pharma 4.0 facilitates process optimizations, efficiency and scalability of products and therapies.
While some newly developed or upgraded industrial applications may still need improvement, in the long run they promise dynamic process optimization and increased levels of agility. At the same time, the increased introduction of single-use technology will allow for more flexibility in terms of seamless adjustments as well as scale-up.
RoSS.KSET as an example for Industry 4.0 in Biomanufacturing
To stick with single-use technology and the benefits thereof: Apart from artificial intelligence, the introduction of single-use systems in bioprocessing has proven to be one of the biggest drivers in pushing the entire industry into a new era.
Novel and bold approaches are the only way to keep improving - especially in times of pandemics. The constantly increasing development speed that leads to the introduction of new products such as individualized therapies at an escalated frequency is another reason why new approaches should not just be dismissed.
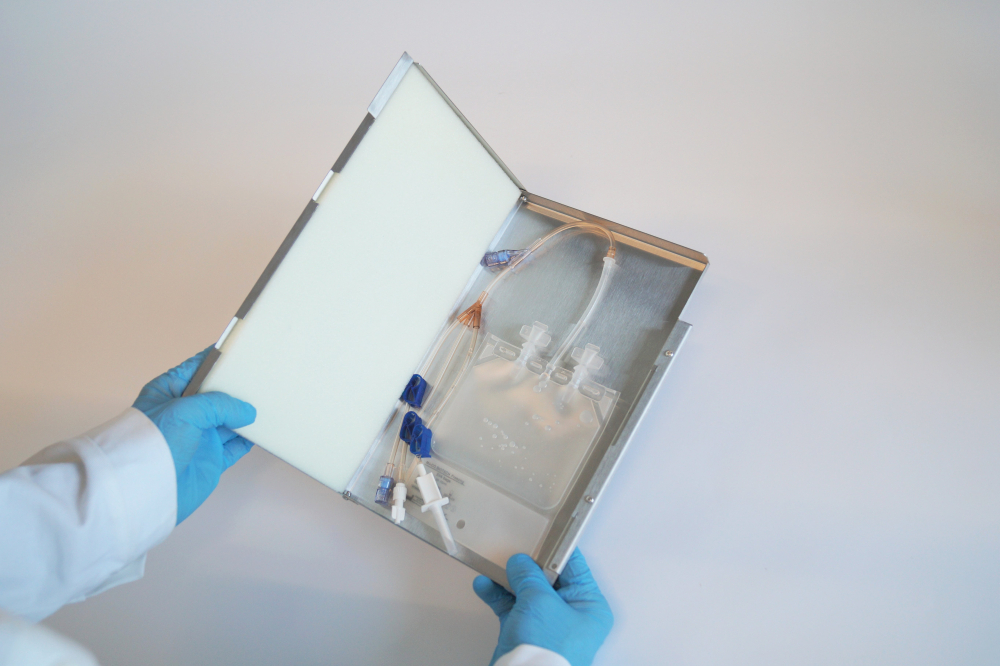
Single Use Support are leading the way in terms of smart manufacturing: Their RoSS.KSET is a best-practice example for what single-use technology can achieve and how easy it is to upgrade to Industry 4.0 respectively Pharma 4.0. The RoSS.KSET is a single-use bag protection for smaller bags. The shell is robust, closed, safe and sterile – most suitable for cell and gene therapies or clinical studies with small volumes of drug substances ranging from 1 mL to 250 mL. It keeps the contents protected from contaminants and other harmful materials and is compatible with a number of different industrial applications and bioreactors.
But where, you may ask, does Industry 4.0 respectively Pharma 4.0 come in here? The answer is relatively simple, as illustrated below:
Track and trace: bringing shipment to the next level
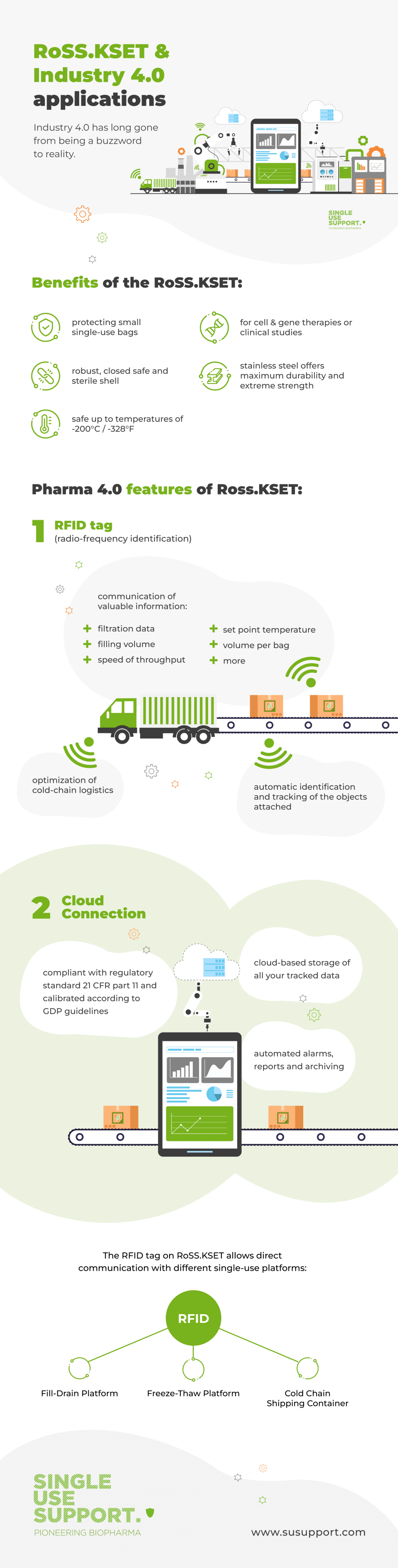
Again, Single Use Support’s RoSS.KSET is a good example: It can be equipped with RFID (radio-frequency identification) tags that use electromagnetic fields to allow for the automatic identification and tracking of the objects they are attached to.
This tag can be embedded in a single-use component, such as the tube or molded connection, or it can be attached to the RoSS.KSET itself to facilitate direct communication with different single-use platforms, such as Fill-Drain-Platform, Freeze-Thaw-Platform or Cold Chain Shipping Container to provide valuable information regarding the biomaterials or biomanufacturing product.
The data includes, but is not limited to, filtration data, filling volume or speed of throughput, set point temperature, or volume per bag. It can be saved for further improvement and optimization of cold-chain logistics and also serves to highlight potential issues - when a freezing process is too slow, for example. By evaluating the automated data, such issues can be addressed and remedied.
Optimization of manufacturing processes
When it comes to autologous cell therapy, time - and traceability - are the essence. But the same is actually true for any highly sensitive matter such as living cells, enzymes or antibodies, as well as vaccines.
This is where Pharma 4.0 can provide help - and improvement - in the form of artificial intelligence; and while single-use technologies themselves are not vital in making Pharma 4.0 work, they can act as vessels.
In addition, RFID also plays an important role in terms of regulatory requirements and good manufacturing practices, as it facilitates transparency throughout the entire biomanufacturing process.
Single Use Support’s smart surveillance technology is compliant with regulatory standard 21 CFR part 11 and calibrated according to GDP guidelines. It allows for the cloud-based storage of all your tracked data and enables automated alarms, reports and archiving, thus allowing you to improve your unit operations.








