What is upstream and downstream processing?
Table of contents
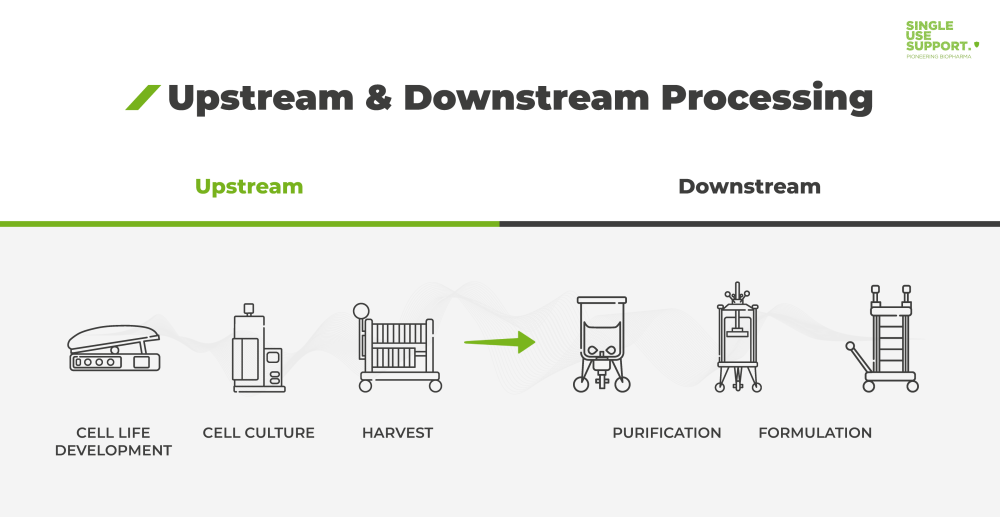
ShowUpstream and downstream processing are two steps inherent to the production of active pharmaceutical ingredients (API) used in biopharmaceuticals. Upstream processing takes small quantities of engineered microbial or mammalian cell culture to grow it to larger volumes in the controlled environment of bioreactors. As the subsequent process step, downstream processing includes the separation and purification - by means of chromatography or centrifugation and filtration - of the cells generated during the upstream bioprocessing step.
The production and processing of APIs is challenged by increasing regulation and manufacturing costs. This calls for flexible solutions throughout the upstream and downstream processing, which is why a growing number of manufacturing processes utilize single-use systems, making the individual process steps scalable from lab to large-scale.
What is upstream processing?
As already mentioned, upstream processing in biotechnology describes the step of preparing cell cultures for fermentation. It encompasses various steps, which will be described in further detail below.
The main aim of upstream bioprocessing, however, is to achieve large-scale cell-growth from small amounts from a variety of cell lines. The required volume can vary, and single-use technologies are an efficient way to cater to different needs and volumes, while titers are used as the primary benchmark to characterize upstream manufacturing efficiency, with higher titers generally indicating that more desired product is manufactured using the same or less amount of fluid or filled bioreactor volume.
Certain parameters, such as glycosylation patterns for monoclonal antibody (mAb) products, are primarily impacted by the upstream process and need to be monitored during the entire process development.
One can differ between two types of upstream bioprocessing in a bioreactor. In perfusion, also called upstream continuous bioprocessing, cell-culture is removed from the bioreactor and replaced with fresh cell-culture media continuously. It was implemented to produce modern biopharmaceuticals where time to market counts. Perfusion is performed for maximum efficiency due to high flexibility, efficient use of facilities and cost reductions which come with it. Whereas in fed batch systems nutrients are fed to the bioreactor during cultivation. Fed-batch reactions typically last up to 14 days.
Steps in upstream processing
The upstream part of a bioprocess refers to the initial stage in which microbes/cells are grown from either bacterial or mammalian cell lines in bioreactors. Cultivation and cell growth can be achieved by adding nutrients, growth hormones or cell culture media to the fermenter. Microbial organisms can grow much faster than mammalian cells, with cultivation times of several days or even hours. Upstream processing involves the following steps, all of which are related to inoculum development:
- Master cell bank (MCB)
- Working cell bank (WCB)
- Media Preparation
- Cell Culture
- Cell Separation
- Harvest and Clarification
Once the cells are ready for harvesting, they will be extracted before being further processed in the downstream processing step. In the clarification filtration steps, the harvested material of a bioreactor is prepared for downstream purification by reducing the content of impurities and particles.
What is downstream processing?
Now that the cells have been cultivated and harvested, they need to be recovered and purified to be of further use for biomanufacturing processes. Downstream processing implies manufacture of a purified final product - including antibiotics, hormones and enzymes - usually procured in large scales, while analytical bioseparation (also achieved by downstream processing) refers to the purification process for the sole purpose of measuring a component or components of a formulation.
The latter may require sample sizes as small as a single cell, while approved vaccines and gene therapy products require larger quantities, which makes the option to scale-up not only desirable; rather, it is viewed as an integral part of process development.
Steps in downstream processing
Downstream processing includes processes required to take biological materials such as cells and derive from them a pure product. The various steps of downstream processing involve:
- Separation
- Cell disruption
- Extraction
- Isolation
- Purification
- Polishing
- Virus filtration/inactivation
- Concentration
Typical operations to achieve the removal of insolubles are filtration, centrifugation, sedimentation or precipitation. Product isolation is the removal of components with properties that vary considerably from that of the desired final product. Isolation steps to remove impurities include solvent extraction, adsorption and ultrafiltration.
Optimization of Bioprocessing
The development and cultivation of monoclonal antibodies (mAbs), gene therapy vectors, and other advanced treatments is a highly specialized process dealing with extremely sensitive biologics, thus requiring sophisticated solutions.
The optimization of bioprocessing unit operations not only yields significant savings but more importantly leads to increased safety and reliability. In addition, bioreactor upgrades and the implementation of single-use technologies can achieve an increased degree of scalability that allows to take different formulations from lab to large-scale production.
But in order to minimize the risk of bio contamination and product loss, optimizing biomanufacturing processes extends beyond the upgrade of upstream and downstream unit operations. Single-use bioprocessing allows for optimization of storage and shipping - it basically covers the entire supply chain.

The optimization of upstream and downstream processes happens on the increasing use of single-use systems. Typical stainless steel bioreactors and tubing are being replaced by smaller, more flexible single-use systems. These offer maximum flexibility, cost savings, scalability, and a small environmental footprint. Single-use equipment is widely used to enable scalable bioprocessing. A study from American pharmaceutical review in 2020 shows that more than 85% of processes in these areas use disposables.1
Safe storage & shipping of APIs
There are still significant technological gaps in the interface between upstream/downstream and fill/finish and within each bioprocessing process. All process steps are in the biopharmaceutical production chain. With these areas often being geographically detached, a reliable, high-level handling of bulk drug substances has to be guaranteed. In terms of viability, single-use technologies are ideal: They offer secure solutions for filling, freezing, thawing and any liquid transfer or logistics processes.

It is vital that APIs have consistent product characteristics, regardless of the production batch or container in which they were collected following final filtration. Variations in the concentration of product- and/or process-related impurities are to be prevented at all costs as they can have an impact on the efficacy of the product, and consequently the patient’s safety. A closed system that can be adapted offers a versatile solution as it can be utilized from early-stage trials and lab purposes all the way to blockbuster production.
Upstream & Downstream processing: FAQs
What are the similarities between Upstream and Downstream processing?
Upstream and downstream processing are unit operations required in the production of biologics, using host cell proteins. By definition, however, the two process steps differ, as upstream deals with inoculum development, while downstream bioprocessing deals with purified, harvested and clarified products and can include final product development.
What is the difference between Upstream and Downstream processing?
The key difference is that upstream bioprocessing involves screening and identification of microorganisms, media preparation, multiplication of microbes inside bioreactors, while downstream bioprocessing deals with extraction, purification and filtration of the resulting product.
What are examples of process steps in downstream bioprocessing?
Downstream processing involves all unit operations after fermentation that improve the purity of the final product. Process steps and unit operations in downstream processing are for example cell disruption, separation, filtration, chromatography, ion-exchange, purification, extraction and clarification, isolation, crystallization, inactivation, concentration, and polishing.
What are examples of applications in upstream bioprocessing?
Upstream processing includes formulation of the fermentation medium, sterilization of air, fermentation medium and the fermenter, inoculum preparation and inoculation of the medium. One of the areas of application is the production of monoclonal antibodies, which are used to express recombinant antibodies. Read more about fermentation in the pharmaceutical industry.