History & Development of ADCs
Table of contents
ShowAntibody drug conjugates (ADCs) are a class of therapeutics that combine the specificity of monoclonal antibodies with the potency of cytotoxic drugs.
Since the first ADCs entered clinical trials in the 1990s, they have shown great promise in treating a variety of cancers and other diseases. However, the development of ADCs has also been marked by challenges and setbacks, including issues with efficacy, safety, and manufacturing.
In this article, we will explore the history of ADCs, from their early development to the latest advances in technology and applications. We will also discuss the current state of the field and the future outlook of antibody drug conjugates, with a focus on ADC manufacturing.
The Early Development of ADCs
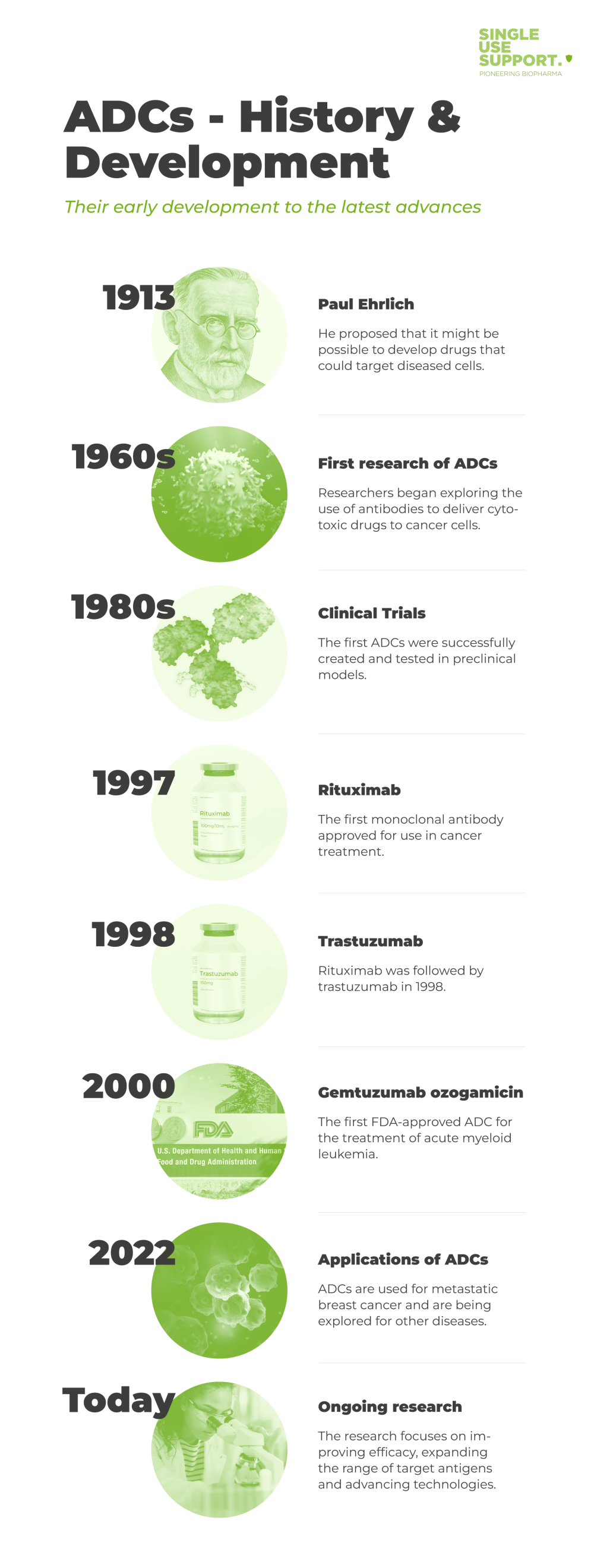
The idea of using antibodies as targeted therapies for cancer can be traced back to the pioneering work of Paul Ehrlich in the early 20th century. He proposed that it might be possible to develop drugs that could selectively target diseased cells without harming healthy tissues.
In the 1960s, researchers began exploring the use of antibodies to deliver cytotoxic drugs to cancer cells. Early examples of this approach included the use of antibodies to deliver radioactive isotopes to tumors.
In the 1980s, the first ADCs were successfully created and tested in preclinical models. However, the development of ADCs was limited by a lack of suitable antibody targets, difficulties in linking antibodies to drugs, and concerns about toxicity and immunogenicity.
Despite these setbacks, the development of ADCs continued, and the technology has since evolved to produce several successful drugs for the treatment of cancer.
Image of Paul Ehrlich
Monoclonal Antibodies and the rise of ADCs
Monoclonal antibodies paved the way for the development of ADCs. The first monoclonal antibody approved for use in cancer treatment was rituximab in 1997, followed by trastuzumab in 1998.
The first FDA-approved ADC (Food and drug administration approval) was gemtuzumab ozogamicin in 2000, for the treatment of acute myeloid leukemia. However, it was later withdrawn due to safety concerns. Despite challenges, several other ADCs have been approved for the treatment of various cancers. 1
Challenges & Setbacks in ADC History
The development of ADCs has been marked by significant challenges and setbacks. One of the major issues is the difficulty in achieving an optimal balance between the potency of the cytotoxic drug and the specificity of the antibody. Another challenge is the potential for toxicity, particularly in non-target tissues. The cytotoxic payload can cause damage to healthy cells, leading to adverse effects and limiting the potential use of ADCs in treating certain cancers.
In addition, there have been concerns about manufacturing and stability of ADCs. The process of bioconjugation can be complex, and there are numerous factors that can affect the stability of the final product. This can impact the efficacy and safety of the ADC.
Furthermore, the cost of manufacturing and development of ADCs is also a challenge. ADCs are complex molecules that require specialized manufacturing processes, and this can drive up costs. Additionally, the lengthy and costly clinical trials required for FDA approval can be a significant barrier for companies seeking to develop ADCs.
New ADC technologies
The challenges faced in the development of ADCs led to the emergence of new technologies that aimed to overcome these obstacles. One of the most significant advances was the use of site-specific conjugation methods that allowed for more precise control over the drug-to-antibody ratio.
Another key innovation was the development of novel linkers that could be cleaved under specific conditions, such as low pH in the tumor microenvironment, reducing off-target toxicity.2
In addition, advances in antibody engineering and selection have enabled the generation of antibodies with improved binding specificity, affinity, and pharmacokinetics, enhancing the potency and efficacy of ADCs.
Overall, these new technologies have helped address many of the challenges faced in ADC development, leading to the approval of several successful ADCs for the treatment of various cancers. As research in this field continues, it is likely that even more advanced and effective ADC technologies will be developed in the future, further expanding the potential applications of these promising therapeutics.
Advances in ADC manufacturing with single-use technology
In addition to advancements in linker and payload technologies, the emergence of new manufacturing technologies has also helped to overcome some of the challenges in ADC development. One notable example is the use of single-use technologies in ADC manufacturing.
Single-use technologies allow for faster and more flexible manufacturing in comparison to stainless steel bioreactors and other equipment that must be thoroughly cleaned and validated between batches. Instead of using large, fixed equipment, single-use systems employ disposable components such as bioreactors, single-use bags and tubing sets. These components are pre-sterilized and can be easily replaced between batches, eliminating the need for cleaning and validation.
Read more: Monoclonal antibody manufacturing process
The use of single-use technologies in ADC manufacturing has several benefits. It can reduce the risk of contamination and improve batch-to-batch consistency. It can also increase manufacturing speed and flexibility, allowing for rapid scale-up or scale-down as needed.
Furthermore, single-use systems can be more cost-effective than traditional stainless steel equipment, as they require less capital investment and maintenance. As a result, more and more self-sufficient companies as well as CDMOs for antibody drug conjugate manufacturing are adopting single-use technologies in their production processes.
Overall, the emergence of new ADC manufacturing technologies such as single-use systems has helped to address some of the challenges and limitations of traditional manufacturing methods, and has enabled the production of high-quality ADCs at a larger scale.

Current and future applications of ADCs
ADCs have shown great potential in treating various types of cancer, and several ADCs have been approved by regulatory authorities for clinical use. Since 2022 ADCs are also used for metastatic breast cancer. In addition to cancer therapy, ADCs are also being explored for the treatment of other diseases such as viral infections and autoimmune disorders.
The development of novel ADC technologies, including site-specific conjugation and dual-targeting strategies, is also expanding the potential applications of ADCs.
The future of ADCs looks promising, with ongoing research focused on improving efficacy and safety profiles, expanding the range of target antigens, and advancing manufacturing technologies to reduce costs and increase production efficiency. ADCs will play an increasingly important role in the field of precision medicine, offering targeted and personalized treatment options for patients.
Conclusion and Outlook for ADC development
In conclusion, the development of Antibody Drug Conjugates (ADCs) has revolutionized the field of cancer treatment. Over the years, extensive research and clinical trials have led to the development of highly potent and specific ADCs, which have shown remarkable efficacy against a variety of cancer types.
The early challenges faced in ADC development, such as low efficacy, poor stability, and off-target toxicity, have been overcome through technological advancements and improved understanding of the underlying biology. The use of single-use technologies in ADC manufacturing has significantly contributed to the development of robust and efficient manufacturing processes, which can produce ADCs with high purity and yield.
Looking ahead, the potential of ADCs as a therapeutic modality is immense, and ongoing research continues to explore new targets and mechanisms of action. With the continued development of single-use technologies and manufacturing processes, the scalability and cost-effectiveness of ADC production will continue to improve, paving the way for wider clinical use and improved patient outcomes. Overall, the history and future of ADCs are exciting, and they hold great promise for the future of cancer therapy.
FAQs about Antibody-Drug Conjugates History
Who invented antibody-drug conjugates?
The concept of antibody-drug conjugates was first proposed by Paul Ehrlich in the early 1900s, but the first ADC was not approved until 2000.
What was the first FDA approved ADC?
The first FDA-approved ADC was gemtuzumab ozogamicin, which was approved in 2000 for the treatment of acute myeloid leukemia. However, it was later withdrawn from the market and then reintroduced in 2017 with new dosing recommendations.
When were antibody-drug conjugates discovered?
Antibody-drug conjugates (ADCs) were first described in the literature in the 1960s, but the concept of using an antibody to deliver a drug to a specific target was not realized until the 1980s. In 2000, the first ADC, Mylotarg (gemtuzumab ozogamicin), was approved by the FDA for the treatment of acute myeloid leukemia.
- The Mysteries of Antibody-Drug Conjugates, https://www.science.org/content/blog-post/mysteries-antibody-drug-conjugates, Published 15.11.2022
- Antibody–drug conjugates: Recent advances in linker chemistry, http://dx.doi.org/10.1016/j.apsb.2021.03.042, Published 2021-04-06