Freezing cells – considerations and solutions
Table of contents
ShowIn the field of life sciences, the cryopreservation of cells plays a pivotal role in advancing research and development. By freezing cells, scientists can safely store valuable cell lines and primary cells, ensuring their long-term storage and viability, e.g. for cell banking. This is especially important in the production of cell-based therapies, where specific cell lines need to be constantly available, but also kept viable via short-term storage in order to be transported to patients in need of cell-based products.
In this article, we will explore the considerations and solutions involved in the crucial process of freezing cells. Nevertheless, it is a key element to successful cell research and development.
Why is cryopreservation of cells important?
Cell cryopreservation is of paramount importance in the life sciences, revolutionizing the way researchers work with cellular material. This crucial technique involves freezing cells at cryogenic temperatures, and offers a host of benefits that have shaped the landscape of modern cell research.
One of the primary reasons cryopreservation is indispensable lies in its ability to maintain cell viability over extended periods, as it would deteriorate at room temperature. By preserving cells in a state of suspended animation, scientists can safeguard their integrity and functionality, ensuring that they remain viable and ready for use whenever needed.
Long-term storage via cryopreservation is also vital in the context of stem cell research. Stem cells, with their unique potential for regenerative therapies and disease modeling, require meticulous preservation to retain their biological characteristics, paving the way for innovative medical advancements.
Moreover, cryopreservation allows researchers to build extensive cell banks, conserving valuable cell lines and primary cells for future experiments and scientific discoveries. These stored resources not only enable reproducibility of experiments but also facilitate collaborative efforts, fueling breakthroughs in various fields of research. But also short-term storage highly depends on cryopreservation, e.g. when cell therapies are delivered to patients that do not find themselves in the immediate surrounding.
Guidelines and protocols in cryogenic cell freezing – what do they include?
Ensuring successful cryopreservation requires well-defined guidelines and protocols. These guidelines are tailored for specific cell types (such as mammalian or bacterial cells) and products, as they aim to ensure the best freezing outcome, including maximum post-thaw viability.
Key considerations in such freezing protocols include:
- Using suitable freezing containers, like cryo-vials or single-use bioprocessing containers, to store cells in liquid nitrogen for long-term preservation
- Selecting the appropriate cryoprotectant, such as DMSO or glycerol, based on the cell type
- Determining the optimal cell density and volume for aliquoting
- Define freezing method to best control cooling rate to prevent cell damage during freezing
In addition, it is essential for manufacturers to work in compliance with cGMP as well as regulatory standards, making sure that the resulting cell-based products are both safe and effective.
Freezing adherent cells or cells in suspension
Freezing adherent cells and cells in culture media present distinct challenges in the cryopreservation process. Adherent cells, attached to a substrate, require additional steps for successful freezing. Prior to freezing, these cells must be detached using enzymes like trypsin or EDTA. The next step is to resuspend cells in suitable freezing media containing cryoprotectants.1
On the other hand, cells suspended in culture media can be directly mixed with the freezing medium for preservation. It is crucial to determine the optimal cell density and volume for aliquoting to maintain cell viability during thawing.
Whether dealing with adherent cells or cells in suspension, careful attention to the freezing process, including the choice of cryoprotectants and cooling rate, is essential to ensure viable and functional cells after thawing.1
Cell freezing process – step by step
Although different protocols may vary, the process of cell freezing usually involves the following steps:
Preparation: It has to be made sure that the cell culture is healthy and at an optimal confluency before calculating the cell density and passage number for proper documentation. For adherent cells, trypsin or EDTA are used to detach them and resuspend in the appropriate culture medium. Cells in suspension are transferred to a centrifuge tube.
Centrifugation: The cell suspension is centrifuged to form a pellet and the supernatant is carefully removed.
Cryoprotectant addition: A freezing medium is prepared, containing a cryoprotectant such as DMSO, FBS or glycerol. The cryoprotectant is slowly added to the cell pellet while gently mixing ensures even distribution.
Homogenization: The cell suspension is homogenized to achieve a consistent cell distribution. This step requires high precision, as even tiny inconsistencies are to be avoided.
Aliquoting: The cryopreserved cell suspension is divided into single-use bioprocessing containers to create appropriate aliquots for future use without repeated freezing and thawing.
Freezing: The sealed containers are placed in a -170 °C freezer to prepare them for cryopreservation, e.g. in liquid nitrogen storage.
Record keeping: Maintain detailed records of the freezing process, including cell type, passage number, freezing date, and storage location.
Necessary equipment
When cells are frozen at cryogenic temperatures, product requirements as well as regulatory standards can only be met with appropriate equipment – some of which will be mentioned below:
Devices
The cell freezing process requires the following essential devices:
- Centrifuge – used to separate cell pellets from the supernatant during cell harvesting, enabling efficient cell concentration
- Homogenizer – necessary to portion cell samples prior to freezing
- Filling platform – aseptic aliquoting in a closed system
- Freezers – a specialized freezer that ensures fast cooling of bags and cryovials, preventing ice crystal formation and maintaining cell integrity during freezing
Reagents and containers
Several key reagents are crucial for successful cryopreservation:
- Cryoprotectants – cryoprotective agents like DMSO or glycerol, added to the freezing medium to safeguard cells from damage during freezing and storage
- Centrifuge tubes – tubes that hold the cell suspensions during centrifugation, facilitating the separation of cells from the supernatant
- Single-use bags and bioprocess containers – specialized containers designed for storing and preserving cryopreserved cells in liquid nitrogen
Best practice – freezing cells with single-use technologies
Embracing single-use technologies in the cell freezing process has become a best practice that offers numerous advantages in the field of life sciences. These innovative technologies provide researchers with practical and efficient solutions to overcome common challenges associated with traditional cell freezing methods.
Single-use technologies provide a high level of flexibility, enabling researchers to handle a wide variety of cell types and volumes. The adaptability of these systems makes them suitable for the freezing of both small-scale and large-scale cell freezing applications, catering to the diverse needs of different research projects.
Single Use Support has, therefore, established a product line-up that accompanies the process of cell freezing, for instance with a dedicated homogenizer and a filling solution that are able to portion even tiny amounts of cells with highest precision.
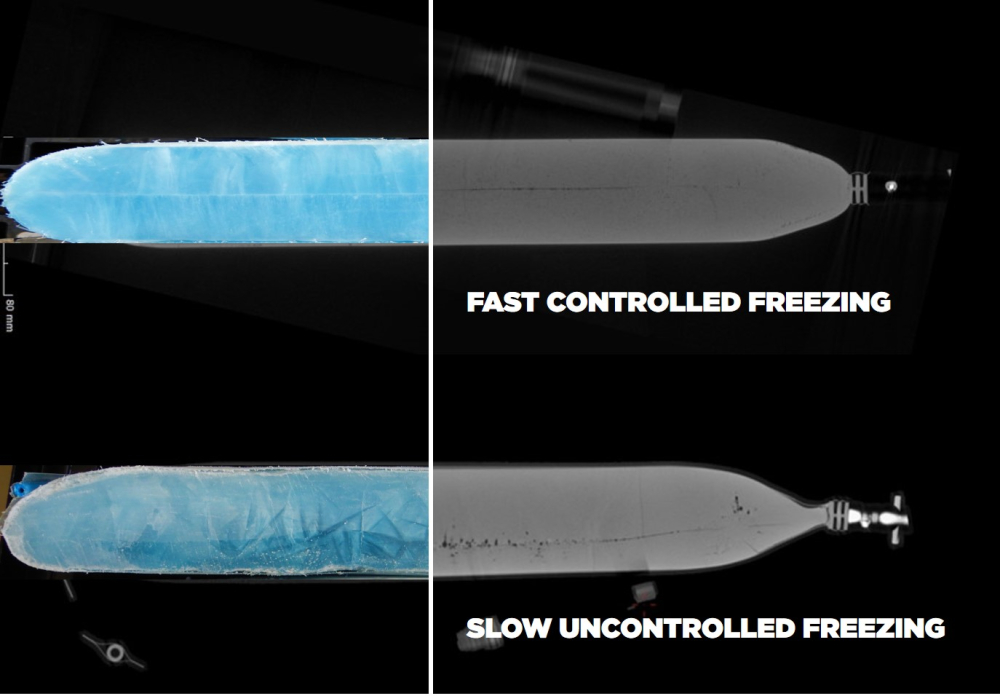
Being filled into single-use bioprocessing containers and covered by protective shells as secondary packaging, cells can undergo a controlled freezing process down to -80 °C with Single Use Support’s plate-based freezing platform. If cryogenic freezing is required, cells can also be frozen at controlled rates with Single Use Support’s RoSS.LN2F, which is the only truly controlled-rate freezer using liquid nitrogen.
Discover the process in detail in our App Note below:
These solutions are designed to facilitate the entire process of cell freezing, allowing researchers and biopharma companies around the world to unleash the huge potentials of cell-based products.
- A Simple and Highly Effective Method for Slow-Freezing Human Pluripotent Stem Cells Using Dimethyl Sulfoxide, Hydroxyethyl Starch and Ethylene Glycol, https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0088696, Published 2014









