Cell and gene therapy: In vivo and Ex vivo in comparison
Table of contents
ShowCell and gene therapy (CGT in short) is a new and promising type of immunotherapy for patients suffering from certain types of cancer and lymphoma/leukemia. It is among the treatments of Advanced Therapy Medicinal Products, in short ATMP.
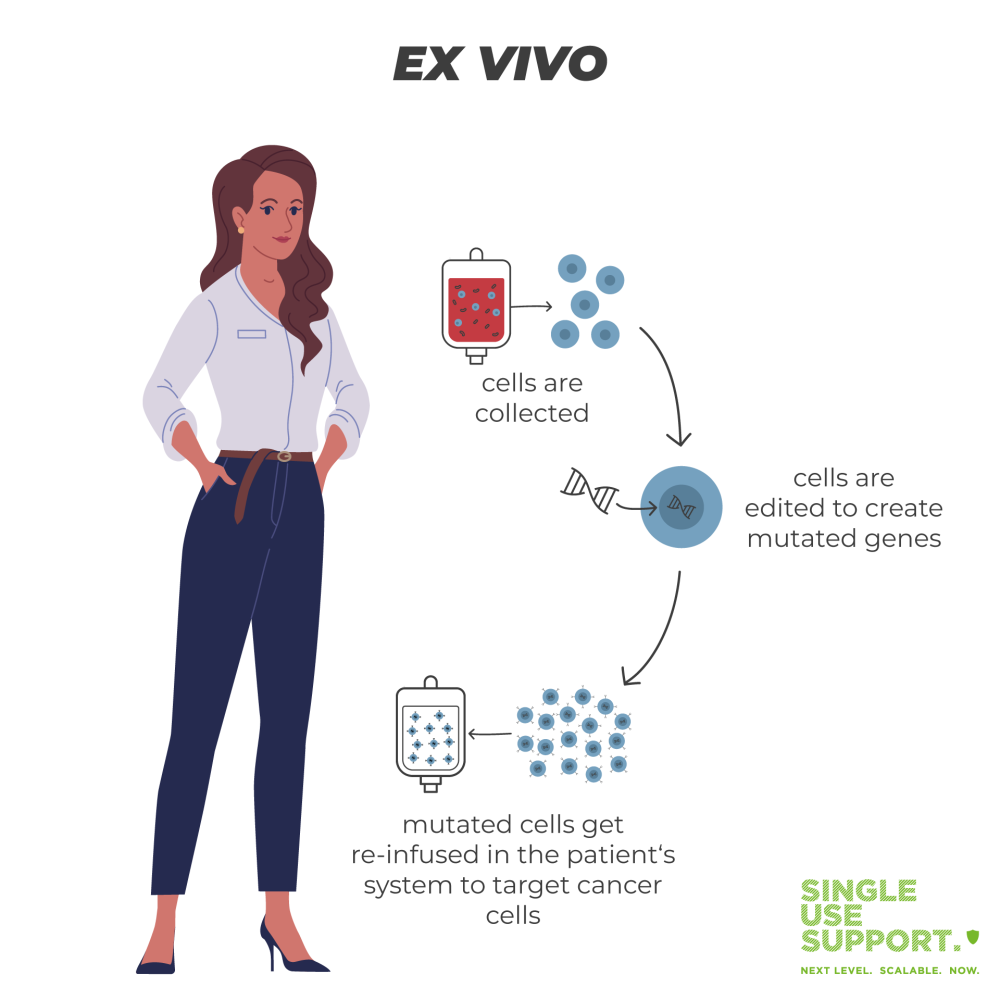
Any ex-vivo type of cell & gene therapy works by utilizing the patient’s own blood cells, respectively genetic material: DNA is extracted to be genetically modified ex vivo. It is then re-infused in the patient’s system to target cancer cells. An in-vivo gene therapy on the other hand is administered directly, with the targeted cells remaining in the body.
Clinical trials have shown breakthroughs for both in-vivo and ex-vivo gene and cell therapy. However, while there are success stories, the full potential of such therapies – both cell and gene therapy – is yet to be explored and realized by biotech experts.
Find out more about the differences between cell and gene therapies as well as in-vivo and ex-vivo and read about genome and gene editing in the following article.
What is gene therapy?
While it was already mentioned that there are two types of gene delivery – in vivo and ex vivo – the question is: What exactly is gene therapy?
Generally speaking, gene therapy is a technique that modifies a person’s genes to treat or cure a disease. It works on the basis of altering (that is introducing, removing, or changing) the content of a person’s genetic code: The alteration of the genetic material by means of genome or gene editing creates mutated genes. The new gene type changes how a single protein or group of proteins is produced by the cell, and is thus an intrinsic part of any gene therapy. Mutations ofgene material can be used to
- reduce levels of a disease-causing version of a protein
- increase production of disease-fighting proteins
- produce new/modified proteins
In short: The altered new gene type can be seen as therapeutic genes, and there is a variety of gene therapy products available. Those include viral vectors (which may be altered to enhance transduction efficiency), nonviral vectors, bacterial vectors, human gene therapy and patient-derived cellular gene products. As already mentioned, gene therapies are not to be confused with cell therapy products, all of which involve the transfer of cells rather than genetic material.
What is cell therapy?
Cell therapy, as opposed to gene therapy, is a type of regenerative medicine that works by using human cells. Stem cells are either taken from the patient or from a suitable donor. After that, they are modified to target cancer cells and certain genetic diseases. After alteration, viable cells are injected or otherwise transplanted into the patient, in a process called immunotherapy. The purpose of immunotherapy is to stimulate the adaptation of the immune system.
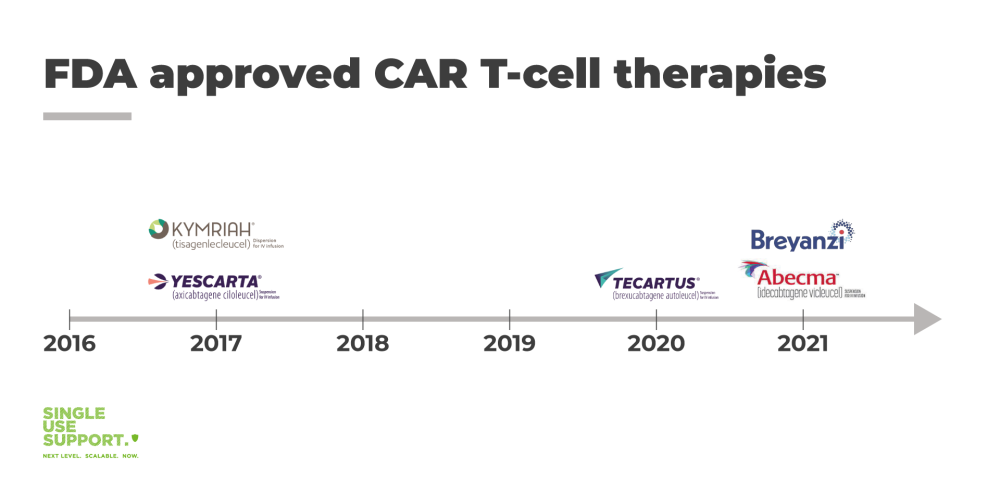
One of the most prominent examples for cell therapies is the CAR T-cell therapy (CAR being short for chimeric antigen receptor). CAR T-cell therapy is a novel treatment promising breakthroughs for patients suffering from certain types of leukemia/lymphoma who are not responding to more traditional approaches (for instance bone marrow transplantation).
There are currently 5 FDA approved therapies. Healthcare organizations worldwide hope for rapid expansion for the benefit of the people.
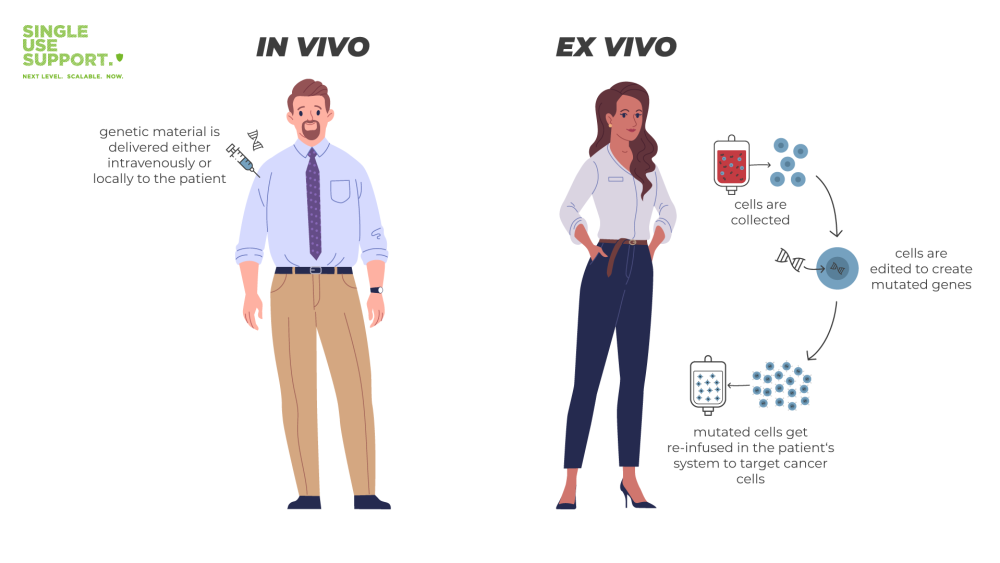
In vivo vs ex vivo approaches
Leaving cell therapies aside and returning to gene therapy, there are two different approaches, namely ex vivo and in vivo. For gene therapy to work, the mutated human gene first needs to get inside the cells of the person affected by the disease. There are two ways to achieve this:
- In vivo meaning: genetic material is delivered to target affected cells (cancer cells or other) that remain inside a person’s body.
- Ex vivo meaning: mutated genes are delivered to a person’s body after the cells have been extracted and exposed to genome or gene editing. Ex vivo cell therapy is also know as gene-modified cell therapy.
Read on to find out what exactly sets these two types of gene therapy apart.
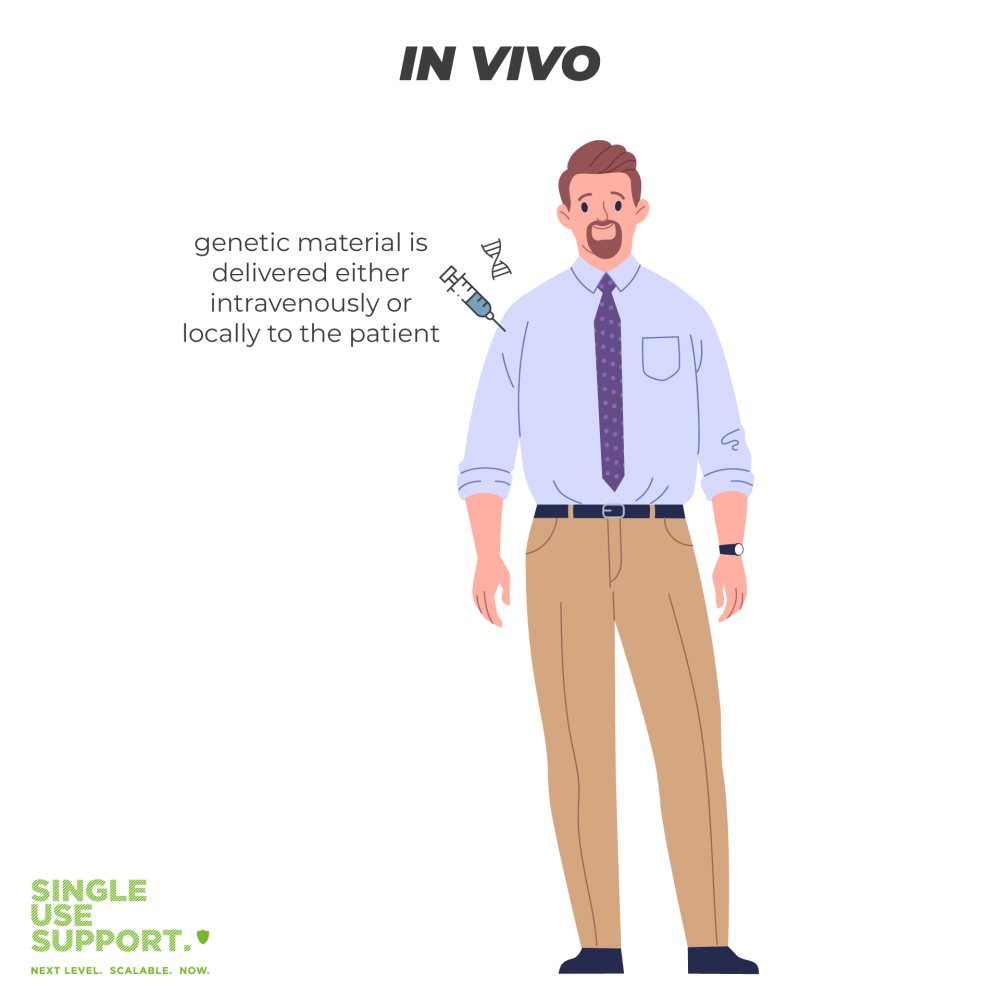
What is in vivo gene therapy?
In vivo gene therapy means that genetic material is directly delivered to the person receiving treatment. This transplantation of genetic material can be done locally to a specific organ or through an IV (intravenous delivery).
The in vivo gene therapy needs the help of a vector, which is typically a virus (e. g. adeno-associated virus, or adenoviruses). A vector passes genetic material into the patient’s cells. The most commonly used delivery system among viral vectors are adeno-associated viral vectors (AAV vectors). Viruses are used as gene delivery vectors because they can efficiently coerce cells into taking up the new gene.
That being said, before any genetic material can be delivered to the patient, it needs to be frozen for safe and protected shipping and storage. Human genes and cells are a sensitive matter, which is why the production of gene therapy products is an elaborate process. In vivo cell or gene therapy requires processes and technologies that are customized to meet the needs of the highly sensitive and valuable human cells and genes. Single-use has proven to be the technology of choice as it allows for flexible and scalable platforms and solutions.
In-vivo cell or gene therapy requires processes and technologies that are customized to meet the needs of the highly sensitive and valuable human cells and genes. Single-use has proven to be the technology of choice as it allows for flexible and scalable platforms and solutions.
What is ex vivo gene therapy?
As opposed to in vivo gene therapy, ex vivo gene therapy refers to a gene editing process. It is common for gene editing technologies to use nuclease-based systems. With the help of such a gene editing technology (e.g. CRISPR/cas9) specific cells are removed from a person to genetically alter and reintroduce them to the patient. This transplantation replaces target cells with manipulated stem cells. This process is most frequently used for
- genetic diseases that affect tissues and organs that can be reached by blood cells
- diseases that affect the blood and immune system.
Ex vivo gene therapy is a good fit for practices in which the corrected cells have a discerning benefit once back in the patient (e.g. for severe combined immunodeficiency) or are easy to obtain. Ex vivo applications are primarily used for disorders which allow the removal, modification and replacement of significant cell population from the patient.
As is the case with in vivo, any altered genetic material needs to be protected, be it from potential contamination or loss of quality. All cell and gene material.
Types of cell and gene therapy
There are several types of cell and gene therapy. While we don’t want to mention all of them, we listed a few to give an overview of how big the variety of possibilities is.
Types of gene therapy:
- Gene addition
Gene addition means that a new copy of a gene is inserted into the target cells in order to produce more of a specific protein. - Gene correction
Gene correction focuses on the modification of a gene part. This process has the goal of removing a faulty or repeated element of a gene. It can also be used to replace dysfunctional or damaged areas of DNA. - Gene silencing
Gene silencing has the goal to prevent the production of a distinct protein. In order to do that, messenger RNA has to be targeted. - Reprogramming
It is often used for diseases in which a tissue, where many cell types are located, is affected. - Cell elimination
Cell elimination means the distinct targeting of tumor cells that are non-cancerous but overly big. It is also known to be used to kill tumor cells which are malignant (cancerous).
Types of cell therapy:
There are many types of cell therapies, but not all of them are yet to be used. Moreover, cell therapies can be differentiated either by the strategy that is used (allogeneic, autologous or xenogeneic) or by its base (stem cell based cell therapy, non-stem cell based cell therapy or multicellular therapy.
One of the most prominent cell therapies is those being blood transfusion (of red blood cells or white blood cells). The most popular one is CAR T-cell therapy. Cells are extracted from the patient’s body and then genetically altered so that they are able to attack cancer cells. It is a very promising, novel therapy in the cure of certain types of leukemia/lymphoma, especially for patients who are not responding to traditional approaches like bone marrow transplantation.
It is not to be confused with stem cell therapy and immune cell therapy.
Further readings: What is autologous? | What is allogeneic?
Challenges & Opportunities in cell and gene therapy
There is general progress in the field of genome and gene editing, and generally regarding cell & gene therapy. Based on results from clinical trials, the US National Cancer Institute is projecting an apparent increase in the number of cancer survivors. Advances in immunotherapy as well as mutated gene and cellular therapy are going hand in hand with significant breakthroughs on the one hand and new challenges for the biotech industry on the other hand.
Personalized CGTs (short for cell & gene therapies) and the involved cell and gene delivery are an extremely cost-intensive approach: Despite the high costs, the yields of genetic material manipulated for cell & gene therapies are low - and supply chains are complex. However, CGT can only be successful if the material modified by genome and gene editing is safely and reliably delivered to the patient.
This is why, once CGTs have passed the phase of clinical trials, the overall aim is a standardization of processes. While there is definitely room for improvement, there have been developments that are already pushing the bar. With single-use technology taking the lead, the goal of standardization - even in the relatively new field of cell & gene therapy, seems to be in closer reach than it ever has been.
In order to achieve this goal, the team at Single Use Support are rethinking existing processes and pioneering innovations around single-use technologies and cell and gene therapy filling system. With their game-changing sterile consumables, platform systems and process solutions for cell and gene therapies, they aim to optimize the entire biotech industry by building upon existing and established technology, like their CGT bag filling machine.