Evolving Aseptic Filling in Biomanufacturing
Table of contents
ShowIn the dynamic landscape of pharmaceutical manufacturing, the journey from laminar flow to automated fluid management has been nothing short of transformative. This evolution, marked by innovation and adaptability, has reshaped the way smaller volumes of biopharmaceuticals are handled and transferred with precision and sterility.
Advances from Laminar Flow to Automated Fluid Management
Decades ago, laminar flow hoods and biosafety cabinets were the stalwarts of aseptic processing, providing controlled environments for fluid transfer tasks. However, as the demand for sterile drug products escalated and regulatory standards tightened, the industry embarked on a quest for more robust and efficient methods.
With advances in advanced therapies came the need to transfer small volumes in an aseptically closed system to reduce the risk of contamination. But it also led to reproducible aliquot consistency, filling accuracy and speed, resulting in improved efficiency in fluid management techniques.
Let's rewind the success story of aseptic filling in the pharmaceutical environment.
Then: Use of Laminar Flow Hood and Biosafety Cabinet
What is a laminar flow hood?
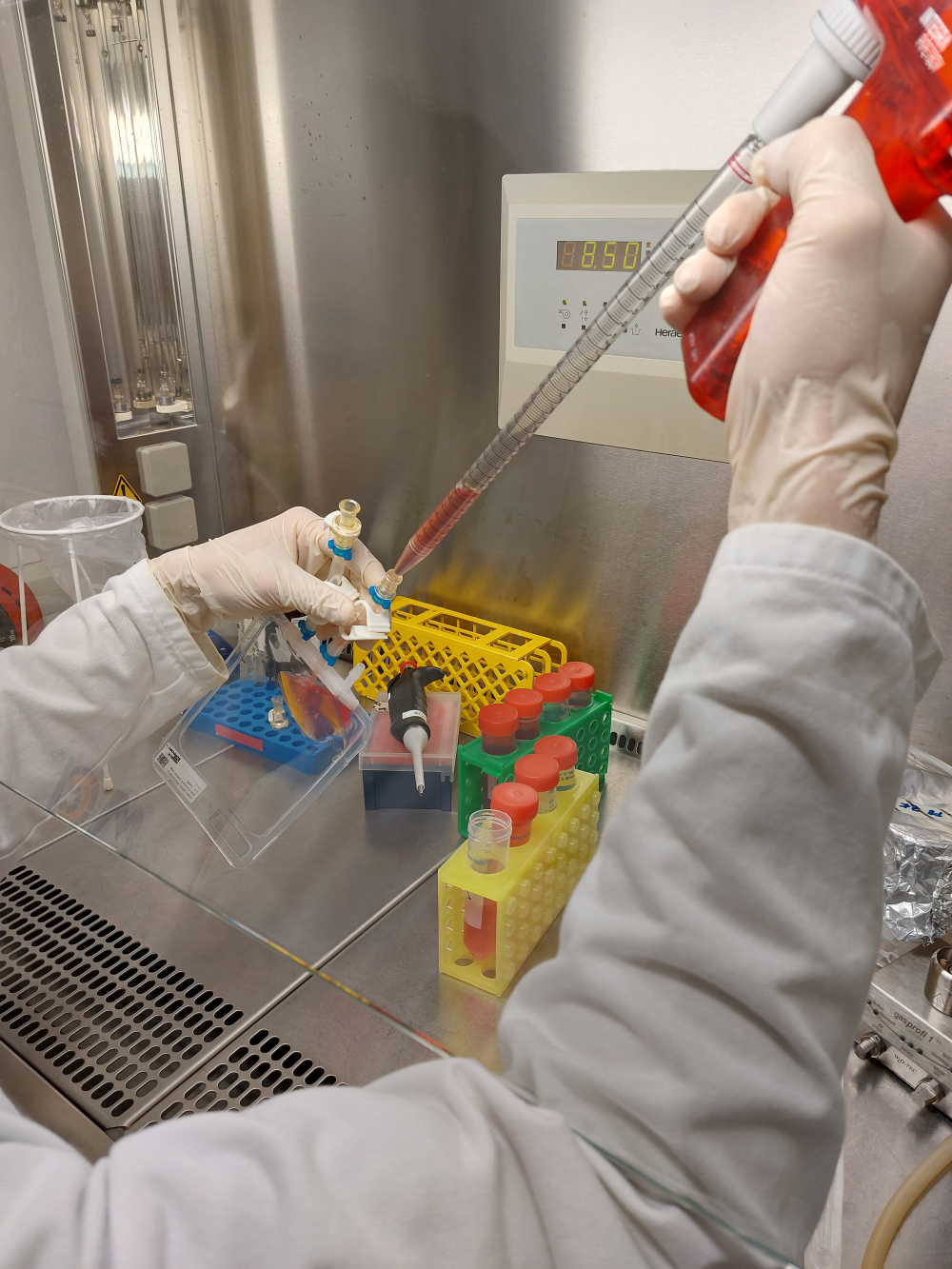
Laminar flow is a technique that creates a unidirectional stream of air with minimal turbulence, ensuring that the air in the work area is free of particles and microorganisms. Laminar flow hoods and biosafety cabinets use this technique to provide a sterile environment for fluid handling tasks, such as filling vials, syringes, or bags with drug substances and drug products. The laminar flow hood consists of a cabinet with a high-efficiency particulate air (HEPA) filter that removes contaminants from the incoming air. The filtered air is then blown across the work surface at a constant speed, preventing any particles or microbes from entering the work area.
What is the difference to a biosafety cabinet?
The biosafety cabinet is similar to the laminar flow hood, but also provides protection for the operator and the environment by enclosing the work area and exhausting the air through another HEPA filter.
Applications of laminar flow hoods and biosafety cabinets
Laminar flow hoods and biosafety cabinets are widely used in pharmaceutical environments where aseptic processing is required, such as compounding pharmacies or research laboratories. These methods enable the manipulation and transfer of sterile fluids without compromising their quality or safety. However, they also require careful adherence to aseptic techniques and sterility requirements, such as wearing sterile gloves, gown, and mask, disinfecting the work surface and equipment, and minimizing the movement and disruption of the airflow.
Laminar flow hoods and biosafety cabinets served as pioneering solutions, offering localized clean environments for fluid handling. Both methods provided controlled environments conducive to aseptic processing. Despite their efficacy in handling smaller volumes, these methods had limitations. They were prone to human-induced contamination despite stringent protocols and lacked the scalability needed for larger operations, which posed challenges in meeting evolving industry demands.
Later: Switch to Filling Line Isolators
The emergence of filling line isolators represented a significant advancement in biopharmaceutical manufacturing. These enclosed systems provided enhanced sterility by isolating the filling process from the external environment.
What are Filling Line Isolators?
Filling line isolators are systems that enclose the filling equipment and the product containers in a sterile chamber, preventing contact with the external environment. The chamber is continuously supplied with filtered air (by HEPA filters) and maintained at a positive pressure to avoid contamination.

There are different ways to perform filling under a Restricted Access Barrier System (RABS). While an active RABS has a HEPA filter and fan unit enclosed inside the barrier framework, a passive RABS uses the existing room HEPA filters.
The operators can access the chamber through gloves or half-suits or use robotic arms to perform the filling process.
Pros and cons of filling line isolators
Filling line isolators are used in pharmaceutical manufacturing to fill liquid products into vials, syringes, single-use bags or bottles. They offered enhanced sterility and reduced contamination risks compared to their predecessors providing manufacturers with advantages including:
- Improved product quality and sterility assurance by reducing human intervention and reducing the potential sources of contamination.
- Risk assessment certifies increased operator safety by providing a physical barrier between the operators and the hazardous products.
- Reduced operating costs by saving on cleanroom space, utilities, cleaning, and validation.
- Increased productivity and efficiency by allowing faster changeovers, higher filling speeds, and lower downtime.
Although they offer improved sterility and reduced risk of contamination, isolators for filling lines have downsides. Insulators
- Are limited in their process flexibility
- Require significant initial investment
- Implies manual processes with a high risk of deviations such as overfilling or underfilling, human operator error and a higher demand on resources such as manpower and operating time.
These disadvantages have hindered widespread acceptance in the industry.
From Isolator to RoSS.FILL
Watch Alexandre Monzo Fuentes in this recorded presentation from Bioprocess International Europe 2024, Vienna, as he discusses scalable single-use technologies to bridge the gap between downstream & fill-finish.
Now: Automated & Scalable Filling Platforms
Today, automated filling platforms stand at the forefront of biopharmaceutical manufacturing, embodying the convergence of robotics, automation, and cutting-edge technology. These platforms offer unparalleled levels of sterility, scalability, and process flexibility, revolutionizing the way fluid transfer processes are executed.

How do Automated Filling Platforms work?
One of the main applications of automated filling platforms in pharmaceutical manufacturing is to perform aseptic filling of single-use bioprocess containers and bottles with liquid products. Aseptic filling is a critical process that requires strict adherence to quality and safety standards, as any contamination or variation can compromise the efficacy and integrity of the product.
Automated filling platforms provide an aseptically closed system that eliminates the need for human intervention and reduces the risk of microbial or particulate contamination. Additionally, automated filling platforms, such as Single Use Support’s RoSS.FILL, enable standardization of the process, ensuring consistent and reproducible results across different batches and sites. By minimizing manual operations, automated filling platforms also reduce the potential for operational errors, such as overfilling or underfilling, that can affect the quality and yield of the product. Furthermore, automated filling platforms offer high accuracy and precision in delivering the required volume and concentration of the product, reducing product loss and improving productivity.
Automated filling platforms represent a quantum leap in biopharmaceutical manufacturing, offering unmatched sterility, scalability, and process flexibility. With minimal human intervention, these platforms streamline operations while ensuring precision and efficiency.
Automated Filling Platform ROSS.FILL
The advantages of automated filling machines extend beyond sterility and scalability. These systems offer enhanced efficiency, reduced product loss, and improved throughput compared to traditional methods. While the initial investment may be high, the long-term benefits far outweigh the costs, making automated filling machines a compelling choice for modern manufacturing facilities.
Advantages of Modular Automated Filling Machines
The advantages of automated filling machines extend beyond sterility and scalability. These systems offer enhanced efficiency, reduced product loss, and improved throughput compared to traditional methods. While the initial investment may be high, the long-term benefits far outweigh the costs, making automated filling machines a compelling choice for modern manufacturing facilities.
Comparison Laminar Flow vs. Filling Line Isolator vs. Automated Filling Platforms
Characteristics | Laminar Flow Hood | Filling Line Isolator | Automated Filling Platform | Comment |
| Reduced Risk of Contamination | × | ⁓ | ✅ | No human operation, no open handling with RoSS.FILL |
| CAPEX | ⁓ | × | ⁓ | One time investment with early amortization |
| OPEX | × | × | ✅ | Reduced resources of workforce required |
| Speed of Operation | × | × | ✅ | Parallel and sequential filling for highest throughput with stepper valves. Automated sealing optional |
| Filling Accuracy | × | × | ✅ | Reproducible accuracy to few mL per bag |
| GMP Annex1 | × | × | ✅ | Recommended fluid path as aseptically closed system. |
| Modularity / Scalability | × | × | ✅ | Scalable aliquotation from 1mL to 1000L+ for 2D bag sizes from 50mL to 50L |
| Footprint | ⁓ | ⁓ | ✅ | Optimized for a maximum volume on the lowest footprint needed |
Advanced Technologies for a Bright Future
The future of aseptic aliquoting and filling in biopharmaceutical manufacturing is bright. Advanced technologies and real-time monitoring systems are poised to further optimize sterility, efficiency, and process control. By embracing innovation and automation, the industry can navigate the evolving regulatory landscape while delivering safe, high-quality medicines to patients worldwide. Pharma 4.0 can drive improved manufacturing processes by leveraging advances with AI with the interplay between advanced technologies.
In summary, the evolution of aseptic aliquoting and filling reflects the industry's relentless pursuit of excellence and innovation. From humble beginnings to automated marvels, the evolution of fluid transfer methods underscores the industry's commitment to quality and patient safety in transferring biologics and advanced therapies.
















