Lyophilization considerations: Comparing freeze-drying to freezing for biopharmaceutical products
Table of contents
ShowBiopharmaceutical products are valuable and often highly sensitive substances. Their active pharmaceutical ingredients (APIs) must be maintained at the highest-possible quality at all times during their production, transport, and storage. This requires sophisticated freezing and fill technologies.
Freeze-drying, also known as lyophilization, is a common process used to preserve certain biopharmaceutical products. However, not all biopharmaceuticals are suitable for freeze-drying. Some products may have specific stability requirements that are better met by alternative preservation methods such as refrigeration or formulation in a liquid state. The decision to use freeze-drying as a preservation method should be made based on the specific characteristics and stability needs of the biopharmaceutical in question.
Biopharmaceuticals that should and should not be freeze-dried
Biopharmaceuticals that are typically freeze-dried include proteins, peptides, and vaccines, such as:
- monoclonal antibodies (mAbs) and antibody fragments
- enzymes
- inactivated vaccines, e.g. hepatitis A vaccines
- subunit vaccines, e.g. influenza vaccines
- recombinant protein-based vaccines, e.g. human papillomavirus (HPV) vaccines
- some viral vector-based vaccines
However, these types of products can also be frozen in a liquid state to achieve reduced product loss and increased process efficiency.

There are ongoing studies into the possibility of freeze-drying lipid nanoparticle (LNP)-based mRNA vaccines, although they are not typically freeze-dried [1]. These vaccines are formulated as LNP complexes that protect and deliver the messenger RNA (mRNA) to cells so that they can carry out protein synthesis. The LNPs play a crucial role in stabilizing the mRNA and facilitating its entry into cells. Maintaining the stability and activity of mRNA vaccines can be achieved through freezing and cold storage at very low or ultra-low temperatures, e.g. between -20°C and -70°C.
Cell-based therapies, which involve the use of living cells as therapeutic agents, are also generally not freeze-dried. These therapies often require the cells to be in a viable state to retain their biological activity and therapeutic potential. Freeze-drying typically involves the removal of water from a product, which can be detrimental to the survival and functionality of living cells. While freeze-drying can be used for certain types of cells, such as bacterial cells and cells of some yeast strains, it is not a commonly used preservation method for living mammalian cells in biopharmaceutical manufacturing due to the potential for cell damage and loss of viability during the process. Cryopreservation remains the preferred method for preserving living cells for biopharmaceutical applications.
Preservation Methods
Lyophilization in Biopharmaceutical Manufacturing
The freezing of biopharmaceutical compounds has generally relied on one of two methods: Lyophilization (also known as freeze-drying) and freezing. Lyophilization involves the removal of water from a product by lowering its temperature and then lowering the pressure so that any water present, now in the form of ice, can be removed by sublimation, i.e. the ice passes directly from the solid to the gas phase [2]. The frozen liquid is pulverized for shipment and storage, before being returned to its original liquid form. During this process step, known as reconstitution, a suitable solvent, usually sterile water or a buffer solution, is added to the lyophilized product.
Although lyophilization is a commonly used method for freezing biopharmaceuticals such as proteins, the lyophilization process itself also exerts stresses on protein molecules that can lead to a reduction in their stability, affecting a product’s quality and safety [3].
Controlled Freeze and Thaw in Biopharmaceutical Manufacturing
Plate-based freezer technology is an approach that provides a more controllable method of freezing APIs. Plate-freezers, such as Single Use Support’s RoSS.pFTU system, ensure controlled and uniform freezing, helping to prevent valuable products from degrading during the freezing process. Plate-freezing has been shown to be effective in the controlled freezing of biopharmaceuticals at the laboratory scale but crucially also at the larger scale that biomanufacturers require. RoSS.pFTU is a scalable platform that can be used for freezing a range of volumes, from small volumes of product in laboratories (RoSS.pFTU Lab Scale) to larger volumes of up to 500L of product (RoSS.pFTU XL). The latter houses ten 50L bags per batch run. Single Use Support’s systems are vendor-agnostic but they can also be used in conjunction with Single Use Support’s RoSS shell secondary packaging range. Therefore, all types and sizes of single-use bags can be used to safely store and transport frozen product.
Controlled freezing refers to controlling the ice front growth speed [4] and the freeze rate [5]. Single Use Support’s plate-based freezing system allows precise control of both these crucial factors, enabling the completely homogenous freezing of samples – an essential requirement when freezing cell suspensions, for example [5]. Controlling the ice front growth speed minimizes the risk of cryoconcentration – a phenomenon that can result in damage to the valuable product being frozen [4]. With some products, a specific freezing rate is required to maximize the preservation of their activity. With plate-freezing, it is possible to control the freezing of 100L of product so that it freezes at a rate of up to 3°C/minute.
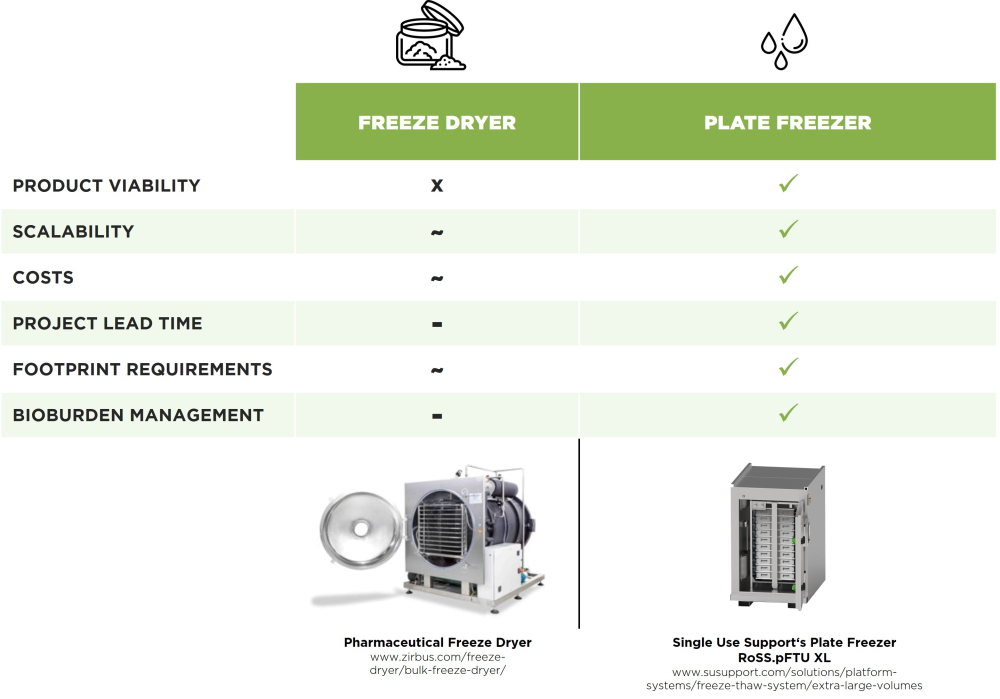
Why Freezing is Smarter than Freeze-Drying
Valuable biopharmaceutical products, such as antibody-drug conjugates, monoclonal antibodies, and mRNA, are often produced in one location but refined and packaged in another location. Therefore, they must be transported in such a way that maintains their quality and minimizes any degradation of their desired properties. This requires carefully controlled and reliable cold-chain logistics, which in turn relies upon the equally carefully controlled process steps of freezing and thawing. Thanks to advanced single-use technologies in pharmaceutical manufacture, product loss through contamination or single-use bag leakages can be minimized toward 0%.
Plate-based freezing shows minimal loss of product quality. The fast and homogeneous freezing possible through plate-based systems and direct surface contact to the protective RoSS® shell prevents the occurrence of cryoconcentration. Additionally, the risk of contamination is reduced when harnessing closed systems for freezing liquids. Plate-based freezers support fast, homogeneous, and safe cooling of APIs, resulting in safer cryopreservation that enables reliable bioburden management and precise control of freezing due to the use of automated systems [4].

With lyophilization, on the other hand, product losses can range from a few percentage points to more considerable losses of 10% or more. These numbers can vary considerably depending on the specific circumstances. What undoubtably has a major impact on product viability is the open handling of freeze-dried biopharmaceuticals until their reconstitution.
Plate-freezers offer scalability, from small to large volumes of bulk drug substances, and are therefore suitable for a wide variety of applications, including the laboratory freezing of a few milliliters to the commercial production of hundreds of liters. Compared with other freezing technologies it involves a lower total cost of ownership and an early return on investment.
References
- International Journal of Pharmaceutics, 2021. 601, 2021, 120586.
- Bakry, A., et al., Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Comprehensive Reviews in Food Science and Food Safety, 2015. 15: p. 143-182.
- Bolje, A. and S. Gobec, Analytical Techniques for Structural Characterization of Proteins in Solid Pharmaceutical Forms: An Overview. Pharmaceutics, 2021. 13(4): p. 534.
- Jenewein, R. and M. Breitrainer, How Controlled Freezing becomes Reality Impact of Ice Front Growth Speed on Scalability of Freezing Protein Solutions (RoSS.pFTU white paper). 2022, Single Use Support.
- Single Use Support, "Bestcellers": Controlled Filling & Freezing of Cells. nd, Single Use Support.









