Automated bioprocessing – 7 advantages of automation
Table of contents
ShowAutomated bioprocessing brings several advantages for the biopharmaceutical industry. Bioprocessing in general involves the use of living cells to produce drugs, vaccines, and other biologics. The biomanufacturing process is complex and requires process control, optimization, and monitoring to ensure product quality.
Automation has revolutionized the bioprocessing industry, enabling real-time control of the manufacturing process, reducing variability, and improving product quality. In this article, we will explore 7 advantages of bioprocessing supported by automation.
Bioprocessing and biopharmaceutical manufacturing
Bioprocessing describes procedures involving living cells, organisms or components to produce biological products like biopharmaceuticals.
The biomanufacturing process includes cell culture, upstream processing, and downstream processing, which includes product recovery, purification, and formulation. The process is complex and requires precise control of process parameters, such as temperature, pH, dissolved oxygen, and nutrient levels, to ensure product quality.
The biopharmaceutical industry has traditionally relied on stainless steel equipment for bioprocessing. However, the use of single-use technology has gained popularity in recent years due to its flexibility and cost-effectiveness. Single-use equipment allows for easy changeover between batches, reduces the risk of cross-contamination, and eliminates the need for cleaning and sterilization.
Advantages of automated bioprocessing
Automation brings numerous advantages to biotechnology, allowing for the production of high yields in excellent quality. In the following, we discuss the 7 most important advantages of automated bioprocessing.
1. Optimized process control
Automation systems enable real-time monitoring and control of the bioprocessing workflow, allowing for precise control of process parameters. This results in improved process control and optimization, reducing the risk of deviations and improving product quality. Automation platforms provide a comprehensive solution for electronic reports and audit trails, providing upstream (USP) or downstream bioprocessing (DSP) process engineers with access to process automation and data analysis in accordance with FDA’s Title 21 CFR Part 11.
2. Enhanced product quality
Automated bioprocessing improves product quality by reducing the necessity for operator interaction and simplifying production processes. Enhanced user-friendliness, streamlined processes and modern technologies can reduce the risk of human error and variability.
For instance, the automated control over freezing parameters and rates as well as real time monitoring reduces the risk for cryoconcentration. Furthermore, automated monitoring allows for early detection of process deviations and enables immediate corrective action. This results in consistent product quality and reduces the risk of product recalls.
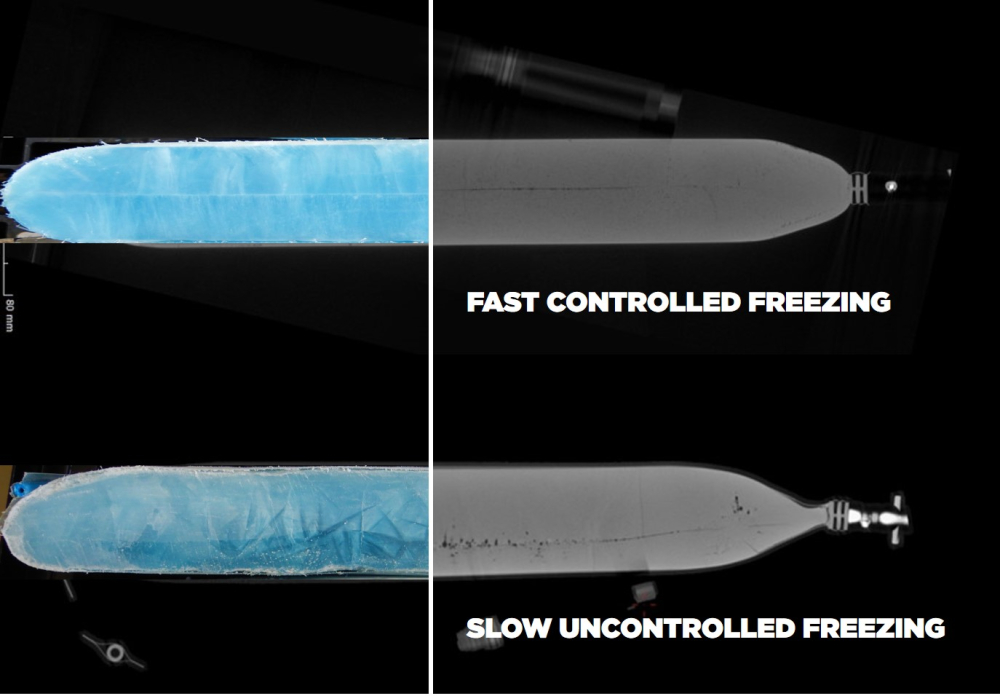
3. Coping with variability
The use of automation in bioprocessing reduces variability by providing consistent process conditions, minimizing the impact of unit operation error, and reducing batch-to-batch variability. Accurate filling with RoSS.PADL as well as homogenous aliquoting with RoSS.FILL help to reduce deviations at every production scale, even for smaller volumes. This results in a more reliable manufacturing process and higher product yield.
4. Increased throughput and efficiency
Automation enables increased throughput and efficiency by reducing the time required for manual tasks and allowing for continuous processing. Single-use bioreactors and downstream processing equipment, such as filtration and chromatography, can be easily integrated into an automated workflow, reducing the time required for changeover between batches.
6. Scalability via bioprocess automation
Scalability is a key requirement in many bioprocesses, when production is brought from lab or small scale to large scale. However, it is important to consider a subsequent scale-up early in the process conception – already at research scale, because although the process might need to be optimized and adapted several times, many issues can be avoided in the first place by thorough planning.1

7. Sustainability with single-use bioprocessing
Sustainability is an essential aspect in process development for the biotech industry. By relying on automated single-use bioprocessing, the environmental impact of the biotechnological industry can be immensely reduced: Single-use technologies do not require elaborate cleaning processes, saving water, energy and time. Additionally, putting adequate waste management into place further reduces the ecological footprint of disposable solutions.
Conclusion: Why automation is key in bioprocessing
Automated bioprocessing provides numerous advantages to the biopharmaceutical industry, including improved process control, enhanced product quality, reduced variability, increased throughput and efficiency, and cost savings. The use of automation in bioprocessing has revolutionized the industry, enabling real-time control of the manufacturing process and improving product quality.
Single Use Support has been contributing to this shift on the biopharmaceutical market, developing end-to-end solutions based on single-use technologies. With scalable platforms for freeze/thaw processes, fill/filtration as well as solutions for storing and shipping drug substances, Single Use Support provides specialized equipment for customers around the world that help to bring their biopharmaceutical processes to the next level.
FAQs
What are the advantages of automated bioprocessing?
The advantages of automated bioprocessing include improved process control and optimization, enhanced product quality, coping with variability, increased throughput and efficiency, and cost savings.
What is downstream processing?
Downstream processing refers to the final stages of biopharmaceutical processing, including product recovery, purification, and formulation.
How does automation improve bioprocessing?
Automation improves bioprocessing by enabling real-time control of the manufacturing process, reducing the risk of deviations and variability, and improving product quality. Additionally, single-use bioprocessing solutions can bring an even higher level of efficiency as well as sustainability.
- Emerging trends and challenges in AAV manufacturing for gene therapies, https://insights.bio/cell-and-gene-therapy-insights/webinars/415/Emerging-trends-and-challenges-in-AAV-manufacturing-for-gene-therapies, Published









