Freezing biologics – best practices
Table of contents
ShowFreezing biologics is an almost unavoidable step in the process of preserving and storing them for an extended period of time. After all, biologics encompass a diverse range of therapeutic products derived from living organisms. They include monoclonal antibodies, protein solutions, and other biotherapeutics – complex formulations engineered to treat various diseases and medical conditions. The freeze-thaw process requires close attention to formulation, cooling rates, and cryoprotective strategies to maintain the stability and efficacy of the drug product.
In the course of this article, we will discuss some frequently used approaches in preserving biologics, along with their strengths and weaknesses. Finally, there will be an introduction of Single Use Support’s solutions to effectively and safely freeze biologics.
Biologics early considerations
Freezing biologics demands the consideration of various critical factors to uphold their integrity and efficacy throughout the process. Some of these considerations include:
Container selection: Vials, cryovessels, or single-use bags – there are numerous types of containers to choose from when freezing biologics. It is important for manufacturers to consider the thermodynamic characteristics of the materials they are made of to ensure that they can endure the stress that may come with extreme temperature gradients while protecting valuable therapies, such as mAbs or mRNA vaccines.
However, container options do not only differ in terms of built materials, but also in regard to their size: The volume of individual units significantly impacts their freezing behavior, thus also the risk for detrimental effects of freezing like unwanted ice crystal formation or protein concentration.
Formulation and cryoprotective agents: Biologics themselves are already highly complex structures, but they are often not frozen alone, but in combination with other substances, such as cryoprotective agents (CPAs or cryoprotectants). Excipients like glycerol and glycol in formulation development play vital roles in mitigating cryoconcentration effects and sustaining the stability of proteins during freezing and thawing cycles.
Freezing and thawing protocols: Cooling rates are a fundamental aspect in achieving desired freeze-thaw profiles and preserving protein stability. It is one of the most decisive steps in unit operation of pharmaceutical freezers, which is why the next chapter will discuss this facet in more detail.1
Hitting the right cooling rate and speed for biological drug substances
Hitting the optimal cooling rate is crucial when freezing biological drug substances. This rate directly impacts various aspects of the process, including ice formation, protein denaturation, and overall product stability.
Since there is no cooling and thawing rate that is considered to be universally ideal, it has to be individualized for each product. This requires thorough stability studies, including characterization of all its components and the kinetics occurring during freezing.
Linked to freezing rates is the time for phase transition as an even more crucial factor for protein stability: It refers to the amount of time it takes for the product to transition from the liquid to the frozen state, and determines effects like cryoconcentration, pH changes, and glass formation.2

Freezing strategies for biologics
Biotechnology knows several freezing strategies for biologics, with some of them being more eligible than others. However, it always depends on the very scope that a pharmaceutical company or CDMO has when subjecting their products to a distinct freezing method, as well as the individual product characteristics.
In the following sections, we will discuss four methods to freeze biologics and biosimilars.
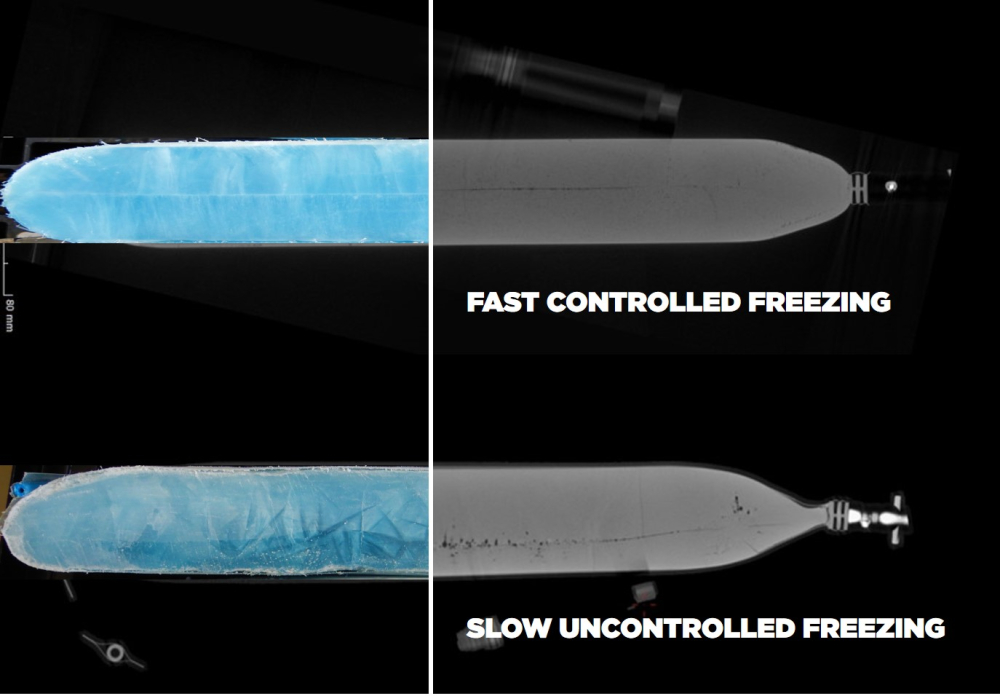
Slow uncontrolled freezing
Slow uncontrolled freezing, characterized by gradual cooling rates and minimal control over ice crystal formation, may appear as a simple approach, but poses significant risks to protein solutions. This method often results in the formation of unwanted ice crystals, inducing denaturation and compromising product stability.
Furthermore, slow freezing brings an increased risk for cryoconcentration, describing an inhomogeneous distribution of protein mass within the sample.3
Lyophilization of biologics
Lyophilization, or freeze-drying, stands as a popular method for preserving biologics, involving freezing the product followed by ice removal under vacuum for extended frozen storage. This process utilizes buffer solutions like sucrose to minimize ice nucleation and stabilize protein formulations.
While lyophilization offers advantages in the preservation of biopharmaceuticals, it also presents disadvantages. These may include high operational costs, longer processing times, and product loss as a result of protein denaturation. Therefore, products like pharmaceuticals based on LNPs are usually not freeze-dried.4
Controlled plate freezing
Controlled plate freezing is a methodical approach to freezing biologics that involves precise regulation of freezing rates and temperature gradients. This technique is not only valuable during process development and small-scale production: With innovative plate freezing and thawing systems like RoSS.pFTU, both small and large-scale freezing of biologics is possible.
By optimizing heat transfer and freezing time, controlled plate freezing ensures the preservation of protein stability throughout the freezing process, with a decreased risk for protein aggregation and other unwanted freezing effects.
Cryogenic freezing involves the use of extremely low temperatures (down to around -180°C) for the preservation of biologics, ensuring long-term stability and viability. This method, integral to cryopreservation, safeguards the integrity of biotherapeutics over extended periods.
Cryobiology plays an important role in individualizing the ideal process parameters and storage temperatures for, e.g., cell therapies, which are typically stored at around -150°C. It represents an essential preservation method in bioprocessing, when ultra-low temperatures (around -80°C) are not sufficient to preserve product quality.
Control over freezing rates, though, has long been a major challenge during cryogenic freezing, due to the use of liquid nitrogen as cooling means. Single Use Support has therefore developed the first truly controlled cryogenic freezer, the RoSS.LN2F, which protects biologics from full exposure to LN2 while following adjustable freezing profiles.
Cryogenic freezing and cryopreservation
Cryogenic freezing involves the use of extremely low temperatures (down to around -180°C) for the preservation of biologics, ensuring long-term stability and viability. This method, integral to cryopreservation, safeguards the integrity of biotherapeutics over extended periods.
Cryobiology plays an important role in individualizing the ideal process parameters and storage temperatures for, e.g., cell therapies, which are typically stored at around -150°C. It represents an essential preservation method in bioprocessing, when ultra-low temperatures (around -80°C) are not sufficient to preserve product quality.
Control over freezing rates, though, has long been a major challenge during cryogenic freezing, due to the use of liquid nitrogen as cooling means. Single Use Support has therefore developed the first truly controlled cryogenic freezer, the RoSS.LN2F, which protects biologics from full exposure to LN2 while following adjustable freezing profiles.5
For cryopreservation of sensitive biologics Single Use Support is convinced of plate freezing and cryogenic freezing as two extremely favorable approaches to control the cooling and thawing process. They can ideally cater to individual product requirements in terms of cooling rates and target temperature, ensuring ideal results upon thawing.
This is why Single Use Support offers innovative solutions for both methods, providing reliable and scalable options for controlled freezing and thawing. The plate-based freeze and thaw technology of the RoSS.pFTU platform ensures homogeneous processing with full control over the process, minimizing the formation of ice crystals and maintaining the stability of biologic substances.
The cryogenic freezer RoSS.LN2F, on the other hand, enables pharmaceutical manufacturers to ensure long-term storage at even lower temperatures, ensuring the viability of biotherapeutics like certain cell-based products – for small and large volumes.
Both systems work with high-quality single-use bags covered in the RoSS® Shell as protective packaging solution. And being based on a modular and vendor-agnostic setup, the solutions by Single Use Support are fully scalable, catering to both small and large scale demands when processing bulk drug substances.
Single Use Support couples the individual strengths of plate and cryogenic freezing with the overall advantages of single-use technologies: They allow for safe and efficient bioprocessing, with elevated flexibility, cost-effectiveness, and sustainability due to a reduction of resource consumption for manual cleaning and sterilization.
Freezing biologics and the advances with single-use technologies
For cryopreservation of sensitive biologics Single Use Support is convinced of plate freezing and cryogenic freezing as two extremely favorable approaches to control the cooling and thawing process. They can ideally cater to individual product requirements in terms of cooling rates and target temperature, ensuring ideal results upon thawing.
This is why Single Use Support offers innovative solutions for both methods, providing reliable and scalable options for controlled freezing and thawing. The plate-based freeze and thaw technology of the RoSS.pFTU platform ensures homogeneous processing with full control over the process, minimizing the formation of ice crystals and maintaining the stability of biologic substances.
The cryogenic freezer RoSS.LN2F, on the other hand, enables pharmaceutical manufacturers to ensure long-term storage at even lower temperatures, ensuring the viability of biotherapeutics like certain cell-based products – for small and large volumes.
Both systems work with high-quality single-use bags covered in the RoSS® Shell as protective packaging solution. And being based on a modular and vendor-agnostic setup, the solutions by Single Use Support are fully scalable, catering to both small and large scale demands when processing bulk drug substances.
Single Use Support couples the individual strengths of plate and cryogenic freezing with the overall advantages of single-use technologies: They allow for safe and efficient bioprocessing, with elevated flexibility, cost-effectiveness, and sustainability due to a reduction of resource consumption for manual cleaning and sterilization.
Read more about biologics
- Winter is coming: the future of cryopreservation, http://dx.doi.org/10.1186/s12915-021-00976-8, Published 2021-03-24
- Large-Scale Freezing of Biologics: Understanding Protein and Solute Concentration Changes in a Cryovessel—Part I., https://www.biopharminternational.com/view/large-scale-freezing-biologics-understanding-protein-and-solute-concentration-changes-cryovessel-par , Published 06/2010
- Shedding light on optimal freezing results for drug substance., https://www.susupport.com/knowledge/freeze-thaw/shedding-light-optimal-freezing-results-drug-substance , Published 05/2021
- Lyophilization considerations: Comparing freeze-drying to freezing for biopharmaceutical products. , https://www.susupport.com/knowledge/freeze-thaw/lyophilization-considerations-comparing-freeze-drying-freezing-biopharmaceutical-products, Published 07/2023
- Cryogenic Freezing: All you need to know., https://www.susupport.com/knowledge/freeze-thaw/cryogenic-freezing-process-biopharma?rel=search , Published 08/2022














