Freeze thaw processes in biosimilar production
Table of contents
ShowIn biosimilar production, freeze thaw processes are indispensable for both intermediates and final drug products. Despite numerous methods being eligible, not all of them can achieve the same levels of quality, safety, and efficiency.
In this article, we will highlight different freezing approaches in biosimilar production, including those Single Use Support recommends as freeze thaw solutions for biosimilars and biologics.
What makes freezing and thawing biosimilars so complex?
Freezing and thawing biosimilars pose unique challenges due to the inherent complexity of these molecules. Unlike small-molecule drugs, biosimilars are intricate biological molecules designed to mimic existing biologic drugs. This complexity arises from their large molecular size, three-dimensional structure, and post-translational modifications.
Maintaining the integrity and efficacy of biosimilars during freeze-thaw processes is critical for ensuring their therapeutic effectiveness. Any deviation from the optimal temperature range or handling procedure can lead to changes of protein stability. These changes can compromise the safety, efficacy, and quality of the final product.
Furthermore, biosimilars and biologics are often more sensitive to temperature fluctuations compared to small-molecule drugs. Even minor variations in temperature or storage conditions can result in protein denaturation, aggregation, or degradation. These alterations may impact the stability and bioactivity of the biosimilar, rendering it less effective or even potentially harmful to patients.
In order to master the complexities revolving around freezing and thawing biosimilars, several approaches have emerged – some of which will be discussed below.
Uncontrolled slow freezing
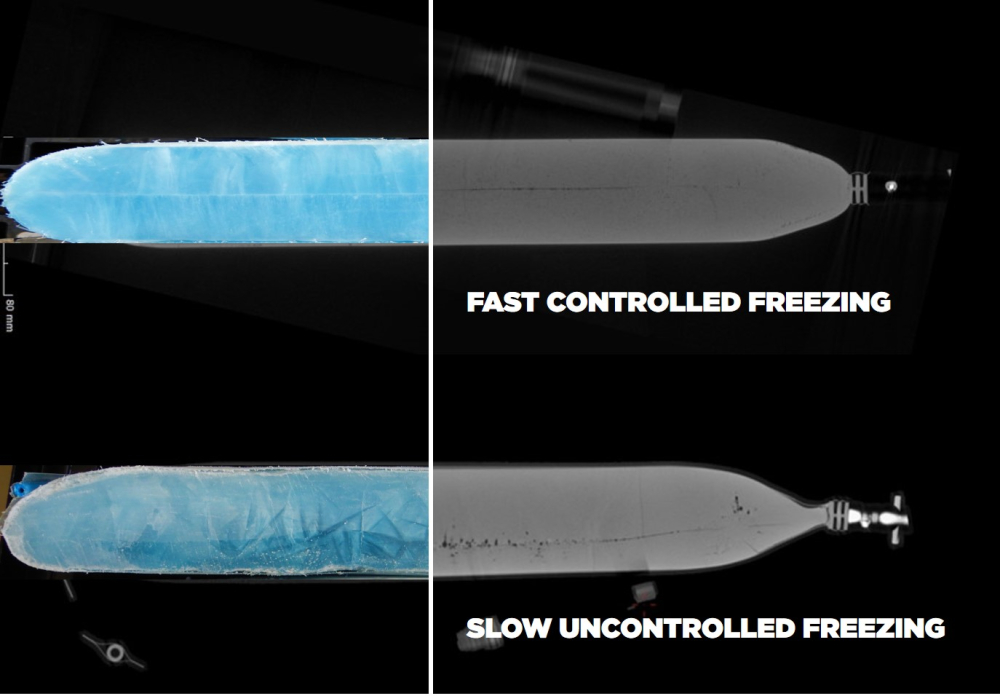
Uncontrolled slow freezing due to cooling with air (e.g. with conventional lab freezers, either upright or chest blast or static freezers) may pose significant risks to the integrity and efficacy of biosimilars during the manufacturing process. Slow freezing refers to the gradual reduction of temperature over an extended period. This process can result in several detrimental effects, including cryoconcentration.
As a consequence, the slow uncontrolled freezing process may not adequately preserve the biological activity of the biosimilar, compromising its therapeutic effectiveness. This is due to a lack of control over freezing rates and the risk for cryoconcentration.1
Lyophilization
Lyophilization, also known as freeze-drying, is a commonly used method for preserving the stability and extending the shelf life of biosimilars. This process involves freezing the biosimilar at low temperatures and then subjecting it to vacuum conditions to remove water by sublimation.
However, lyophilization can be a time-consuming and expensive process, requiring specialized equipment, larger footprint and higher risk of product loss. It may come with an elevated potential for microbial contamination and longer reconstitution times, leading to inconveniences in certain formulations. Additionally, it may not be eligible for all kinds of biosimilars, as it brings significant stress to protein structures. While, under certain circumstances, this approach may, e.g., be eligible for some types of monoclonal antibodies, LNP-based mRNA vaccines and cell therapies using living cells, for instance, are usually not freeze-dried.
Despite its challenges, lyophilization remains a valuable technique for ensuring the stability and viability of biosimilar products.2 3
Plate freezing in biosimilar production
Plate freezing is a method used in the freezing of biosimilars that offers precise temperature control and uniform freezing rates. In this process, biosimilar solutions, filled into bags or other bioprocess containers, are placed on metal plates that are cooled to the desired temperature using a refrigeration system. The plates provide a large surface area for efficient heat transfer, ensuring rapid and uniform freezing of the biosimilar solution.
Plate freezing prevents the formation of undesired ice crystals, minimizing damage to the biosimilar molecules and preserving their integrity and activity. Furthermore, it is a scalable and cost-effective alternative to conventional methods, offering improved product quality and consistency.
Cryogenic freezing
Cryogenic freezing is an advanced method used in the preservation of biosimilars that involves ultra-low temperatures below -150°C (-238°F). This technique utilizes pressured gases, such as liquid nitrogen or liquid helium, that has the capability to rapidly freeze the biosimilar solution. The extremely low temperatures achieved during cryogenic freezing are necessary for the storage of biologics and biosimilars like certain cell therapies, including gene-modified cell therapies.
Cryogenic freezing is recommended when preserving the biological activity and stability of biosimilars over long periods. However, cryogenic freezing has often been “too effective”, meaning that the cooling process occurred too fast and with insufficient control over freezing rates, resulting in intracellular ice formation and hence higher occurrences of cell death. In recent years, though, novel cryogenic freezers have entered the market that address this issue and provide enhanced control during cryogenic freezing.4
What about thawing biosimilars?
Thawing biosimilars means reverting them to their liquid state, which is just as important as freezing them in the first place. And just as intricate, since control over the thawing process is equally vital for the freezing outcome.
In order to meet the individual cold chain requirements of protein substances, it is necessary to provide greater control over thawing rates. Controlled thawing of drug substances ensures standardized processes, as opposed to uncontrolled thawing, where items are simply removed from the fridge and brought to a warmer environment, such as water baths.
Facing freeze-thaw challenges with single-use solutions
A great deal of the challenges in manufacturing biosimilars, especially revolving around freezing and thawing them, comes from the sensitivity of the proteins they are composed of. However, there are also technological limitations to stand in the way of maximum efficiency in biomanufacturing.
Conventional freezing technologies are often either not as scalable or as precise as necessary. Furthermore, widespread needs for human intervention may increase the risks of human error, ultimately leading to product loss.
Still, there are solutions based on single-use technology on the market that address these very problems.
Product loss in biomanufacturing – a bitter pill to swallow?
Product loss in biomanufacturing is a significant concern, impacting both the efficiency and profitability of the process. It can occur either due to loss of product quality, as mentioned before, and therefore limited production yield. But a loss can also occur due to various other factors, including breakage or leakage of single-use bioprocess containers during freezing, shipping, and storage. Excessive manual handling and a missing secondary packaging may be reasons for single-use bags to break. Such vulnerabilities can lead to a loss of valuable drug substances, causing financial setbacks and delaying production timelines.
To address this challenge, innovative solutions like the RoSS® Shell offer a robust secondary packaging option. By providing a protective shell around single-use bags, RoSS® Shell minimizes the risk of breakage or leakage towards 0% – as shown in our case study linked below. This solution not only safeguards the integrity of the bioprocess containers but also ensures the preservation of valuable drug substances throughout the biomanufacturing process.
Powerful freezers for total control
Achieving precise control over freezing processes presents a significant challenge in biomanufacturing. The delicate nature of biological substances, such as monoclonal antibodies (mAbs) (mAbs) and other biopharmaceuticals, demands meticulous handling to maintain their efficacy and integrity. Traditional freezing methods often lack the necessary precision and consistency, leading to potential product loss and compromised quality.
To address this challenge, advanced freezing technologies like the plate freezing platform RoSS.pFTU and the cryogenic freezer RoSS.LN2F offer robust solutions. The RoSS.pFTU leverages plate-based freezing to ensure uniform and controlled freezing of drug substances, maintaining their original quality throughout the process.
Similarly, the RoSS.LN2F cryogenic freezer provides an innovative approach to achieve extremely low temperatures. By utilizing an enclosed LN2 system, this freezer ensures safe and efficient freezing down to temperatures as low as -180°C. With precise temperature control of exposure to liquid nitrogen, the RoSS.LN2F offers unmatched reliability and stability for freezing high-value biopharmaceuticals.
Freezing pharmaceutical bulk: Preparing for scale-up
Scalability is another critical consideration for freezing processes in biosimilar production – but one that, at some point, is inevitable for many manufacturers. There are numerous reasons why these considerations are best made early on in process development, as the necessary equipment requires significant investments. Modular and scalable solutions have therefore entered the market, being able to smoothly transition from small to large scale.
Scaling pharmaceutical freezing, though, does not only refer to an increasing number of individual items to be processed, but also to their respective volumes. Freezing pharmaceutical bulk comes with its own set of challenges, such as achieving homogeneous freezing results.
Single Use Support has made these considerations while developing its freeze-thaw platform based on single-use technologies. Their modular platform design allows for flexible expansion, accommodating varying batch sizes and production volumes with ease, while transferring freezing protocols to larger units.
By using the plate freezing platform RoSS.pFTU, for instance, the direct contact between cooling plates and the packaging surface allows controlled and even freezing processes for various volumes – from 1 ml up to 500 L, depending on the chosen system. This scalability ensures that freezing processes can evolve in tandem with production demands, minimizing disruptions and maximizing productivity.
Scalability was not only a core idea at the conception of plate and cryogenic freezers, but rather for all process solutions developed by Single Use Support – such as fluid management solutions for biosimilar production, storage and transport systems. This enables manufacturers to establish highly automated biomanufacturing processes with minimal need for human intervention, enhanced safety and cost efficiency.
Recommended Articles
- Impact of Freeze/Thaw Process on Drug Substance Storage of Therapeutics, http://dx.doi.org/10.1016/j.xphs.2017.03.019, Published 2017-03-24
- Strategies to Reduce Reconstitution Time of Lyophilized Biotherapeutics, http://dx.doi.org/10.1016/j.xphs.2020.02.019, Published 2020-03-02
- Lyophilization considerations: Comparing freeze-drying to freezing for biopharmaceutical products, https://www.susupport.com/knowledge/freeze-thaw/lyophilization-considerations-comparing-freeze-drying-freezing-biopharmaceutical-products, Published 07/2023
- Cryopreservation as a Key Element in the Successful Delivery of Cell-Based Therapies—A Review, http://dx.doi.org/10.3389/fmed.2020.592242, Published 2020-11-26