E-Book
Aseptic aliquotation and cryopreservation of drug substances are crucial process steps in bioprocessing. Even though they’re not in the spotlight of biomanufacturing.
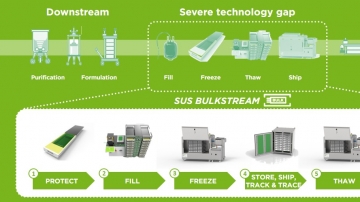
In drug development and drug delivery, biologics must be transferred at numerous occasions. Along the journey of monoclonal antibodies, vaccines and more, the drug substance passes lots of process steps where liquid transfer is required. This is relevant for the transitions between upstream processing, downstream processing and fill & finish, but also within these manufacturing milestones.
The market of fluid & cold chain management has great growth potential and is on its way to professionalization and industrialization. To meet the challenges of manufacturing drug substances, Single Use Support helps manufacturers and CDMOs to advance their fluid & cold chain management process to achieve a secure and efficient process for liquids on the basis of single-use bags.
E-Book
The RoSS® shell offers a robust & safe protection for bioprocess containers of any size and vendor during freezing, transportration, storage & thawing.
With more than 300,000+ RoSS® shells sold worldwide, the RoSS® shell provides a tamper-evident, contamination-free system. The perfect composition for robust storage & shipping for all 2D single-use bags.
The RoSS® shell's composition of elements that embed the bioprocess containers is simple, easy to recycle, validated for use in cleanroom environment, and it offers significant cost-efficiencyand process flexibility. It provides validated robustness, ensures immbolization and enables plate freezing, leading to numerous advantages in biopharmaceutical manufacturing.
E-Book
Single Use Support has a proven track record to provide sterile single-use assemblies to many biopharmaceutical companies in different scenarios:
- Production of thousands of single-use manifolds weekly as a workbench solution during pandemic-driven unavailabilites of single-use components
- Pandemic preparedness campaigns to stock diverse manifolds for future unexpected events
- Ramping up complexity and quantity of single-use assemblies for vaccine production from clinical phase to commercial production throughout a product's lifecycle.