August 2, 2019
Single-use vs. stainless steel technology in biopharma
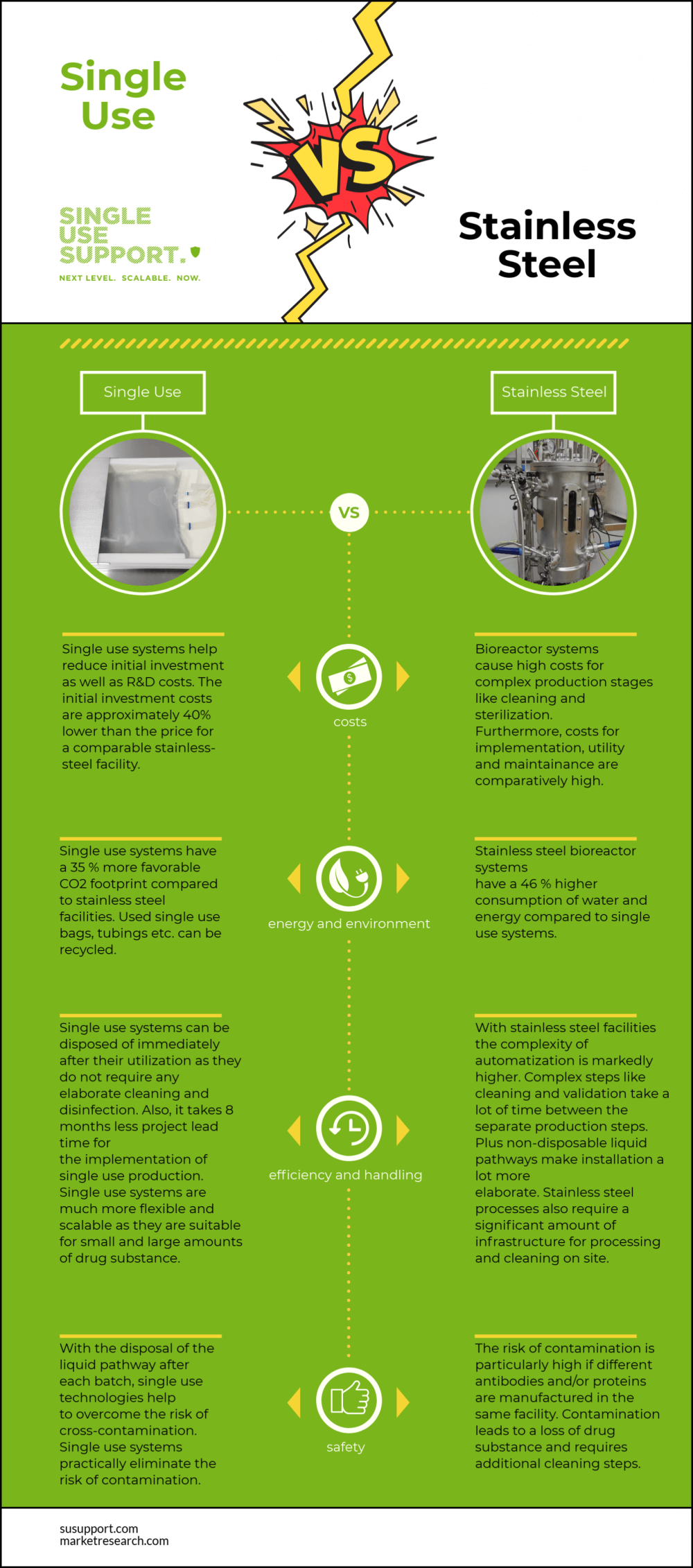
It is not always easy to decide whether to utilize single-use or stainless steel in biopharma. Single-use systems offer a number of advantages over traditional stainless steel systems for the production of biologics, so it is no surprise that they have become a widely accepted standard in the biopharma industry and life sciences in general – be it clinical trials for cell therapy or gene therapy products or related to mass cultivation of cell cultures.
So, why should a biopharmaceutical manufacturer adopt single-use technology (SUT or SUS) in their manufacturing facilities? Here are some major considerations for biotechnology!
Benefits of single-use technology in bioprocessing
1. Reduced cleaning requirements
Stainless steel components and pipework must be repeatedly steam cleaned (SIP or steam-in-place); however, single-use equipment can be pre-sterilized by the supplier using gamma irradiation, and is replaced after each batch. Thus, the use of single-use bioreactors instead of stainless steel bioreactors, for instance, reduces cleaning and validation requirements and, ultimately, leads to lower costs.
2. Decreased plant footprint and capital investment
Stainless steel processes require a considerable amount of infrastructure to be present on site for processing and cleaning, usually referred to as clean-in-place systems (CIP). SUT eliminates the need for a costly stainless steel process and associated utilities plant for cleaning and steaming.
3. Improved batch turn-around times
Quicker batch turn-around times increase a facility’s throughput. With SUT, as soon as a batch is processed, a new pre-assembled and sterilized fluid flow path is installed and ready to process the next batch with no associated downtime for cleaning.
4. Increased process flexibility
Plastic or silicone tubing can be modified more easily and quickly than stainless steel pipework. This is particularly beneficial for contract manufacturing organizations (CMOs/CDMOs) as it allows them to change their manufacturing processes more readily and safely compared to traditional methods.
5. Reduced risk of product cross-contamination
Since the product flow path is discarded and replaced after each batch, the risk of product cross-contamination between batches of different drug substances, related to campaign changeover, is virtually eliminated when using disposable consumables for their pharmaceutical manufacturing processes. Additionally, the risk of microbial contamination during biomanufacturing processes is minimized.
Challenges of single-use systems
Limits in scalability mean that SUT is more suited to small-scale production. Large-scale manufacturers – for which flexibility is of less importance compared to managing the supply chain – may find it more difficult or not cost-effective to completely adopt SUT, although disposable technologies can still be integrated into a process where appropriate.
Plastics used in single-use systems can leach compounds under certain conditions. Single-use equipment providers must therefore provide extractable and leachables data to show that undesirable substances will not enter the drug product stream from components.
Quality control is another issue which must be addressed.
With traditional stainless steel equipment, the responsibility of quality control is managed on site, but with SUT this falls to the supplier. Therefore, appropriate systems must be put in place to ensure that suppliers provide single-use components that meet cleanliness standards.
Sustainability issues – SUT vs steam cleaning
The sustainability issue of disposing of plastics after a single-use has raised environmental concerns. However, when compared to super-heated steam cleaning, which requires energy, cleaning chemicals and large amounts of water, single-use technology is arguably no less sustainable than stainless steel technology.
Safety – or better yet, solidity – single-use requires upgrades!
Of course there is no such thing as the perfect Jack-of-all trades. However, in times of CAR-T, single-use technology and its advantages should hardly be questioned any longer. And there is not shortage in terms of innovations.
Companies like Single Use Support GmbH from Austria have tackled the challenge to expand established single-use elements by means of technological add-ons as well as shell systems for all batch sizes, while maintaining the flexibility and at the same time warranting maximum security and protection, impact resistance and the content’s quality.
Is single-use technology only suitable for small batches?
The answer is a clear no – even if Cryovessel manufacturers like to claim the opposite, which comes as no surprise; after all, their raison d’être rests on increasingly unsound footing while the modular single-use shell systems developed by Single Use Support GmbH are gaining more and more traction, not least thanks to their expandability by Fill & Drain, Filtration automation as well as Freeze & Thaw systems. These solutions also allow an easy scale-up to higher titers and reduce the overall cost of goods, which is beneficial both for biotech companies and healthcare providers.
Checkout MITS.2D – mobile integrity testing system for single-use bags:

Single-use systems have become increasingly popular over the past few years and they are now an established standard in the biopharmaceutical industry. As you can see, the advantages of single-use systems far outnumber those of traditional steel reactors, such as lower operating costs and an easier unit operation in downstream processing like chromatography. So why are some pharma manufacturers – e.g. in the field of monoclonal antibody production – still hesitant when it comes to switching from stainless steel facilities to single-use technologies?
Often the main deciding factor is scalability. While there are single-use systems with capacities of up to 2000 l, handling such massive single-use bioprocess containers might prove difficult. As a consequence, many manufacturers of biopharmaceuticals fear damaging their bags.
Stainless steel tanks, as single-use bags protection, pose no such risk. On the other hand, the contamination rate is substantially higher. Additionally, cleaning and sterilization can be an issue; the same is true for wrongly positioned seals or liquid pathways.
However the case may be, new developments should never be ignored; after all, new technologies that allow for a more effective production process are key to staying competitive.
Single Use Support offers biopharmaceutical companies a new and reliable logistics process for liquids on the basis of standardized single-use bags from all manufacturers.
Sources: Parker and SUSupport.com