E-Book
eBook: Filling Gaps in Managing Large Volumes of Biologics
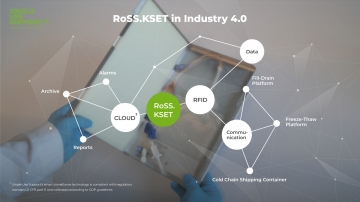
Aseptic aliquotation and cryopreservation of drug substances are crucial process steps in bioprocessing. Even though they’re not in the spotlight of biomanufacturing. In drug development and drug delivery, biologics must be transferred at numerous occasions. Along the journey of monoclonal antibodies, vaccines and more, the drug substance passes lots of process steps where liquid transfer is required. This is relevant for the transitions between upstream processing, downstream processing and fill & finish, but also within these manufacturing milestones. The market of fluid & cold chain management has great growth potential and is on its way to professionalization and industrialization. To meet the challenges of manufacturing drug substances, Single Use Support helps manufacturers and CDMOs to advance their fluid & cold chain management process to achieve a secure and efficient process for liquids on the basis of single-use bags.