Bioprocessing
As expert for cold chain handling in bioprocessing, Single Use Support is providing solutions to meet challenges for safe storing and shipping associated with right freezing and thawing methods and temperature control for long-term storage. Their development approach leads to innovative systems that enable manufacturers to replace manual processes with fully automated end-to-end processes that not only push efficiency but also increase output.
Alexander Fuchs
March 14, 2023
Bioprocessing
Microbial fermentation is a hot topic not only in the biopharma industry. In this article, you will find a brief overview of microbial fermentation manufacturing before we discover different CDMOs offering microbial cGMP production.
Michael Mühlegger
March 9, 2023
Bioprocessing
Pharmaceutical manufacturing processes, including pharmaceutical fermentation, are still prone to human error and product loss. The challenges can be overcome by moving to automated processes that eliminate the need for slow and error-prone manual handling. Adopting automated end-to-end solutions based on single-use technologies minimizes, if not eliminates, human error and product loss.
Daniel Tischler
February 28, 2023
Bioprocessing
Fermentation in the pharmaceutical industry is used to cultivate microorganisms for antibiotics, therapeutic proteins, enzymes and insulin. It typically involves temperature-controlled tanks, also known as fermenters and the correct concentration of nutrients to cultivate the desired organism. Microbial and bacterial fermentation technology and the associated processes open new possibilities and are important building blocks for gene-editing, conjugates and DNA plasmids used in modern vaccine production.
Daniel Tischler
February 17, 2023
Bioprocessing
Precision fermentation uses microbial hosts to produce a particular end product. Other terms used for precision fermentation are bacterial and microbial fermentation.
Precision fermentation is used in a variety of ways in both the food industry and pharmaceutical industry to produce biopharmaceuticals. Read more!
Michael Eder
February 14, 2023
Bioprocessing
Microbial fermentation is a biochemical process that manages to extract chemical energy from carbohydrates without the oxygen.
This chemical reaction occurs in bacteria, yeasts or even in muscles of humans. Read more details!
Michael Eder
February 14, 2023
Bioprocessing
Microbial fermentation in bacteria, yeast or fungi is, due to its benefits, preferred in the production of smaller biologics. These include peptides, proteins, cytokines, growth factors, plasmid DNA, single-domain antibodies, peptibodies and more. Continue reading...!
Daniel Tischler
February 14, 2023
Bioprocessing
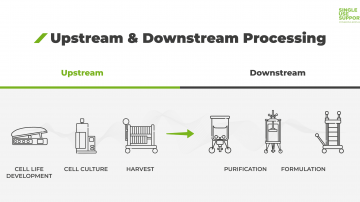
The manufacturing process of biopharmaceuticals such as monoclonal antibodies, mRNA and bioconjugates is divided into upstream and downstream processing. Manufacturers seeking reliable automation and optimization fit for scale-up are increasingly employing single-use bioreactors, titers, filtration and other components, especially during upstream.
Daniel Tischler
December 20, 2022
Bioprocessing
One would think that a sector like the fast-paced biomanufacturing industry could not get any faster. Yet, increased speed-to-market and ever more efficient manufacturing continue to drive product and process development. This is why single-use technologies have become increasingly popular. Read more about single-use systems for downstream processing in this article.
Daniel Tischler
December 19, 2022
Bioprocessing
Upstream and downstream processing are two steps inherent to the production of active pharmaceutical ingredients (API) used in biopharmaceuticals. The production is challenged by increasing regulation and manufacturing costs. This calls for flexible solutions, which is why a growing number of manufacturing processes utilize single-use systems
Daniel Tischler
December 19, 2022
Bioprocessing
Single-use systems are looking ahead a bright future – but what is it that manufacturers need to consider before the switch? Learn more about it in this article!
David Seifert
September 15, 2022
Bioprocessing
Advanced cell-based assay workflow with CryoLogyx Thaw and Test pre-plated cell and controlled, scalable freezing process solution RoSS.pFTU to achieve effective cryopreservation of cells.
Brian Moloney
August 9, 2022