Biopharmaceutical Products
For drug substances that undergo a freezing process during manufacturing, thawing at some point is only a logical consequence. However, there are several factors to be considered for this seemingly easy procedure – find out more in this article!
Michael Eder
March 8, 2023
Biopharmaceutical Products
Freezing large volumes of drug substance can be a challenging endeavor. In this article, we will discuss main considerations as well as possible solutions for this delicate process.
Alexander Fuchs
March 7, 2023
Biopharmaceutical Products
The antibody-drug conjugates (ADCs) market is expected to grow significantly in the next few years. But what are the key players in this competitive landscape? Find out more in this article!
Michael Eder
November 7, 2022
Biopharmaceutical Products
Antibody-drug conjugates might revolutionize the treatment of cancer and various other diseases. However, there are some challenges to overcome in their handling, including controlled freezing – find out more in this article!
Johannes Kirchmair
October 13, 2022
Biopharmaceutical Products
Viral vectors are looking ahead a bright future in gene therapies. In this article, we give you an overview of the most frequently used viral vectors and have a glimpse at their possible future applications.
Daniel Tischler
October 11, 2022
Biopharmaceutical Products
A great example is the treatment of insidious cancers with CAR T-cells, that relies on modifying the patient’s own T-cells to instill them with a targeted way to fight cancer cells. Learn more why forming strong collaborations will solve many issues!
Michael Mühlegger
October 10, 2022
Biopharmaceutical Products
Mastering the complex CAR T manufacturing process and the associated supply chain challenges in the cell and gene area are the major bottlenecks during the large-scale commercialization of highly promising CAR T cell products.
Michael Eder
June 23, 2022
Biopharmaceutical Products
mRNA vaccines require high diligency along their supply chain. This is why every step in the mRNA vaccine supply chain must be carefully planned, carried out in a safe and controlled manner and requires appropriate, innovative technologies.
Daniel Tischler
June 9, 2022
Biopharmaceutical Products
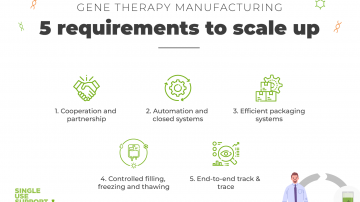
Gene therapies have become a highly promising field of groundbreaking next generation treatment, but there are some challenges to face within the manufacturing process. Here are 5 factors that will be crucial for upscaling gene therapy manufacturing with single-use technology.
Michael Mühlegger
June 9, 2022
Biopharmaceutical Products
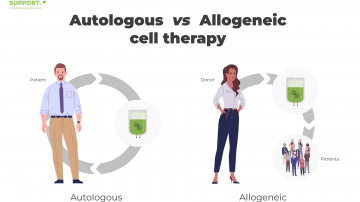
Although autologous and allogeneic cell therapies share both defining methodological key elements as well as various fields of application, including most innovative cancer therapies, there are significant differences between them. Therefore, a closer look on both procedures shall be presented.
Brian Moloney
May 18, 2022
Biopharmaceutical Products
In order to allow for a smooth and functioning process, a reliable supply chain, tailored to the specific needs of autologous therapies, is a prerequisite. Single Use Support has developed an end-to-end process solution for cell and gene therapies.
Michael Eder
January 10, 2022
Biopharmaceutical Products
Single Use Support is solving problems related to supply chain management through the development of smart packaging and related smart technologies for CAR T-cell therapy. Read more!
Brian Moloney
December 20, 2021